��Ŀ����
��2009?��������������AlN���������¡�������������Ժõ��������ʣ����㷺Ӧ���ڴ��ģ���ɵ�·�������մɹ�ҵ������
��1������ԭ�ӽṹʾ��ͼΪ��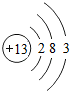 ����ԭ�Ӻ�������������������1������ԭ�ӵ�������Ϊ
����ԭ�Ӻ�������������������1������ԭ�ӵ�������Ϊ
��2���������մ����ڣ�ѡ����ţ�
���л��ϳɲ��� �ڽ������� �����ǽ�������
��3����ҵ���Ƶ��������Ǵ��������л��������X��ͨ��������Һ�����������������õ�N2��X��N2��̼��һ�������·�Ӧ�Ƶ�AlN��CO��������X�Ļ�ѧʽΪ
��1������ԭ�ӽṹʾ��ͼΪ��
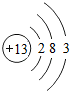 ����ԭ�Ӻ�������������������1������ԭ�ӵ�������Ϊ
����ԭ�Ӻ�������������������1������ԭ�ӵ�������Ϊ14
14
����2���������մ����ڣ�ѡ����ţ�
��
��
�����л��ϳɲ��� �ڽ������� �����ǽ�������
��3����ҵ���Ƶ��������Ǵ��������л��������X��ͨ��������Һ�����������������õ�N2��X��N2��̼��һ�������·�Ӧ�Ƶ�AlN��CO��������X�Ļ�ѧʽΪ
Al2O3
Al2O3
����AlN�Ļ�ѧ����ΪAl2O3+N2+3C�T2AlN+3CO
Al2O3+N2+3C�T2AlN+3CO
����������1����������ԭ�ӽṹʾ��ͼ��֪��������������ݴ˿��жϺ�����������
��2�����ݲ��ϵķ��������ɣ�
��3�����ݷ�Ӧ�������д����Ӧ�ķ���ʽ�����������غ㶨���ƶ����壮
��2�����ݲ��ϵķ��������ɣ�
��3�����ݷ�Ӧ�������д����Ӧ�ķ���ʽ�����������غ㶨���ƶ����壮
����⣺��1��������ԭ�ӽṹʾ��ͼ��֪�������������13����֪�������������14��
��2���ɲ��Ϸ����֪ʶ��֪���������մ��������ǽ������ϣ�������ɷ��в����л���ͽ��������Բ����ڽ������Ϻ��л��ϳɲ��ϣ�
��3����������X��N2��̼��һ�������·�Ӧ�Ƶ�AlN��CO���������غ㶨�ɿ�֪������X�Ļ�ѧʽ�� Al2O3��������������������̼��һ�������·�Ӧ�Ƶ�A1N��CO����ѧ����ʽΪ��Al2O3+N2+3C�T2AlN+3CO��
�ʴ�Ϊ����1��14����2���ۣ���3��Al2O3��Al2O3+N2+3C�T2AlN+3CO��
��2���ɲ��Ϸ����֪ʶ��֪���������մ��������ǽ������ϣ�������ɷ��в����л���ͽ��������Բ����ڽ������Ϻ��л��ϳɲ��ϣ�
��3����������X��N2��̼��һ�������·�Ӧ�Ƶ�AlN��CO���������غ㶨�ɿ�֪������X�Ļ�ѧʽ�� Al2O3��������������������̼��һ�������·�Ӧ�Ƶ�A1N��CO����ѧ����ʽΪ��Al2O3+N2+3C�T2AlN+3CO��
�ʴ�Ϊ����1��14����2���ۣ���3��Al2O3��Al2O3+N2+3C�T2AlN+3CO��
���������⽫��ѧ֪ʶ�����������ϵ�����������������д����л�ѧ������ʱ���������⣬�������⣬���������йصĻ�ѧ֪ʶ�л�����ϵ���������ɽ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ