��Ŀ����
�̵�������װ����̽���֣������ã��ƽ�ǽ𣬲������ɻ�ͭ�������ģ��ҹ��Ŵ��ϱ���ʱ�ھ��������ͭ����ͭ��ͭ��п�ĺϽ����������������������������ճ���Ʒ��Ϊ�˲ⶨij��ͭ��Ʒ��ͭ������������ȡ20g�û�ͭ��Ʒ�ӵ�50gϡ�����У�ǡ����ȫ��Ӧ����������0.2g������ͭ�Dz���ϡ���ᷢ����Ӧ�ģ�������1���û�ͭ��Ʒ��ͭ������������
��2��ϡ������Һ�����������������
���𰸡�����������ͭ����ϡ���ᷢ����Ӧ�����������0.2g�ǻ�ͭ��п��ϡ���ᷴӦ���ɵģ����������������뻯ѧ��Ӧ����ʽ�ɼ���п�������������
����⣺��1����ǡ����ȫ��Ӧʱ��ͭ��п������Ϊxg����������Ϊyg��
��Zn+H2SO4=ZnSO4+H2��
65 98 2
x y 0.2g
 ��
��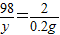
���x=6.5g��y=9.8g
��أ�Cu��%=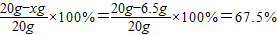 ��
��
�𣺸û�ͭ��Ʒ��ͭ����������Ϊ67.5%��
��2����50gϡ�����к�������9.8g����
�أ�H2SO4��%=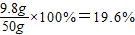 ��
��
��ϡ������Һ���������������Ϊ19.6%��
����������ϼ�����ѧ�����û�ѧ��Ӧ����ʽ�ļ��㣬������������ֱ�Ӵ���п�����ᷴӦ�ķ���ʽ�м���һһ���
����⣺��1����ǡ����ȫ��Ӧʱ��ͭ��п������Ϊxg����������Ϊyg��
��Zn+H2SO4=ZnSO4+H2��
65 98 2
x y 0.2g
 ��
��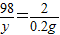
���x=6.5g��y=9.8g
��أ�Cu��%=
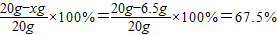 ��
���𣺸û�ͭ��Ʒ��ͭ����������Ϊ67.5%��
��2����50gϡ�����к�������9.8g����
�أ�H2SO4��%=
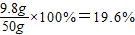 ��
����ϡ������Һ���������������Ϊ19.6%��
����������ϼ�����ѧ�����û�ѧ��Ӧ����ʽ�ļ��㣬������������ֱ�Ӵ���п�����ᷴӦ�ķ���ʽ�м���һһ���

��ϰ��ϵ�д�
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д�
�����Ŀ