��Ŀ����
ˮ��Դ�ı����ͺ��������Ѿ��ܵ����ǵ��ձ��ע�������������������и���
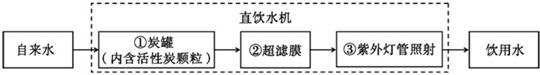
��1����Щ����ȡ���ǵĺ�ˮ��������ˮ�������ǵĺ�ˮ����ͼ1��ʾ�ļ���ˮ�����о��������л���̿��������________
�ô�װ�þ�����õ���ˮ����________����������������
��2���þ�ˮ����ͬλ�õ�ɴ���������Ҫ���ò�ͬ������С��ʯ��ʯӢɳ֮���ɴ���������ǣ�________
��3����Щ���ȡ�õ���ˮ������ij����ˮ��Ӳˮ������ˮ�����õ�������________��
��4��ˮ�����츣���࣬��ˮ����Ⱦ��ȴ������������ѣ�Ϊ�˷�ֹˮ����Ⱦ�����и��������ˮ�����ж���ֲ����������ڲ������ŷŹ�ҵ��ˮ���۽�ֹʹ��ũҩ�ͻ��ʣ���������ˮ�����������������ŷţ����п��Բ��õķ�����________
A���٢�B���ڢ�C���٢�D���ڢ�
��5������Ӧ�����ᳫ��Լ��ˮ������д��һ�ֽ�ˮ��ʩ________
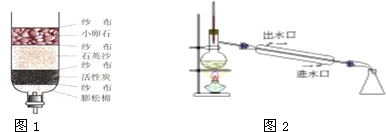
��6��ʵ����Ϊ��������Һ��������ͼ2��ʾ��װ����ȡ�����̶Ƚϸߵ�����ˮ��
�������Ӻ�װ�ú�Ӧ�Ƚ���________���IJ������ټ���ˮ�ͷ�ʯ������������ƿ�м����ʯ��Ŀ����________��
�⣺��1�������ǵĺ�ˮͨ������ˮ�����о��������л���̿������������ˮ�е�ɫ�غ���ζ���ô�װ�þ�����õ���ˮ����Ȼ����һЩ����ˮ�����ʣ����ڻ����������������
��2���þ�ˮ����ͬλ�õ�ɴ���������Ҫ���ò�ͬ������С��ʯ��ʯӢɳ֮���ɴ���������ǽ�С��ʯ��ʯӢɳ���룻�����С��ʯ��ʯӢɳ���룻
��3����ˮ�м������ˮʱ�����������������ĭ��������ˮ�������������ĭ���٣�����Ӳˮ���������ˮ��
��4���������ŷŹ�ҵ��ˮ��������ˮ�����������������ŷſ��Է�ֹˮ��Ⱦ��������ˮ�����ж���ֲ��������ͽ�ֹʹ��ũҩ�ͻ����Dz���ʵ�ģ�����D��
��5���ÿڱ���ˮˢ����ʹ�ý�ˮϴ�»�����ϴ��ˮ�����ȿ��Խ�Լ��ˮ������ÿڱ���ˮˢ����ʹ�ý�ˮϴ�»�����ϴ��ˮ�����ȣ�
��6����������Ҫ���װ�õ������ԣ�����ƿ�м����ʯ��Ŀ���Ƿ�ֹҺ�屩�У�������װ�õ������ԣ���ֹҺ�屩�У�
��������1������̿�����������ã��ܹ�����ˮ�е�ɫ�غ���ζ��ͨ�����ˡ������ȷ������ܳ�ȥ����ˮ�����ʣ�
��2���þ�ˮ����ͬλ�õ�ɴ���������Ҫ���ò�ͬ������С��ʯ��ʯӢɳ֮���ɴ���������ǽ�С��ʯ��ʯӢɳ���룻
��3���÷���ˮ���Լ���ˮ��Ӳ�ȣ���pH��ֽ���Բⶨ��Һ�����ȣ�
��4�����ݷ�ֹˮ��Ⱦ��;�����н��
��5��Ҫ��������Լ��ˮ����Ҫ�ԣ�
��6��������������Ҫ���װ�õ��������Լ�����ƿ�м����ʯ��Ŀ���Ƿ�ֹҺ�屩�н��н��
�����������Ҫ���վ���ˮ�ķ����ͽ�Լ��ˮ����Ҫ�ԣ�ֻ���������ܶ���ط��������������ȷ���жϣ�
��2���þ�ˮ����ͬλ�õ�ɴ���������Ҫ���ò�ͬ������С��ʯ��ʯӢɳ֮���ɴ���������ǽ�С��ʯ��ʯӢɳ���룻�����С��ʯ��ʯӢɳ���룻
��3����ˮ�м������ˮʱ�����������������ĭ��������ˮ�������������ĭ���٣�����Ӳˮ���������ˮ��
��4���������ŷŹ�ҵ��ˮ��������ˮ�����������������ŷſ��Է�ֹˮ��Ⱦ��������ˮ�����ж���ֲ��������ͽ�ֹʹ��ũҩ�ͻ����Dz���ʵ�ģ�����D��
��5���ÿڱ���ˮˢ����ʹ�ý�ˮϴ�»�����ϴ��ˮ�����ȿ��Խ�Լ��ˮ������ÿڱ���ˮˢ����ʹ�ý�ˮϴ�»�����ϴ��ˮ�����ȣ�
��6����������Ҫ���װ�õ������ԣ�����ƿ�м����ʯ��Ŀ���Ƿ�ֹҺ�屩�У�������װ�õ������ԣ���ֹҺ�屩�У�
��������1������̿�����������ã��ܹ�����ˮ�е�ɫ�غ���ζ��ͨ�����ˡ������ȷ������ܳ�ȥ����ˮ�����ʣ�
��2���þ�ˮ����ͬλ�õ�ɴ���������Ҫ���ò�ͬ������С��ʯ��ʯӢɳ֮���ɴ���������ǽ�С��ʯ��ʯӢɳ���룻
��3���÷���ˮ���Լ���ˮ��Ӳ�ȣ���pH��ֽ���Բⶨ��Һ�����ȣ�
��4�����ݷ�ֹˮ��Ⱦ��;�����н��
��5��Ҫ��������Լ��ˮ����Ҫ�ԣ�
��6��������������Ҫ���װ�õ��������Լ�����ƿ�м����ʯ��Ŀ���Ƿ�ֹҺ�屩�н��н��
�����������Ҫ���վ���ˮ�ķ����ͽ�Լ��ˮ����Ҫ�ԣ�ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��3����ͼ���þ�ˮ��Ͱ������оʵ��ͼ���������ѧ֪ʶ�ش��������⣮
��3����ͼ���þ�ˮ��Ͱ������оʵ��ͼ���������ѧ֪ʶ�ش��������⣮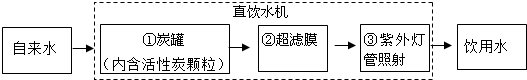
 �����ҹ����ϵ����������أ�ˮ��Դ�ı����ͺ����������ܵ����ǵ��ձ��ע���������������������й����⣺
�����ҹ����ϵ����������أ�ˮ��Դ�ı����ͺ����������ܵ����ǵ��ձ��ע���������������������й����⣺