��Ŀ����
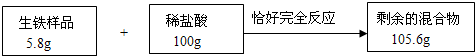
ij����С��Ϊ�˲ⶨͭ���Ͻ�������������������ȡͭ���Ͻ���Ʒ20g����80gϡ����ƽ���ֳ�4�ݣ���4�����뵽��Ʒ�У�ÿ�γ�ַ�Ӧ����ʣ������������±���
����
��1��20gͭ���Ͻ��У�ͭ������Ϊ
��2����
��3��20gϡ������Һ�����ʵ���������Ҫ��д���㲽�裩
| �� �� | 1 | 2 | 3 | 4 |
| ����ϡ��������/g | 20 | 20 | 20 | 20 |
| ʣ���������/g | 17.2 | 14.4 | 11.6 | 11.6 |
��1��20gͭ���Ͻ��У�ͭ������Ϊ
11.6
11.6
g����2����
3
3
�κϽ��е���ȫ��������ȫ����3��20gϡ������Һ�����ʵ���������Ҫ��д���㲽�裩
����������ϡ����ֻ�ܺ�ͭ���Ͻ��е�����Ӧ�����ÿ��20gϡ���ᣬ������������������Ϊ���������������ɱ������ݷ�����֪������ͼ�����ݿ�֪����1��2��3�η�Ӧ��������ʼ��ٵ�������Ϊ2.8g������4�μ���20gϡ����ʱ�����������ټ��٣�˵��ͭ���Ͻ�����ȫ�����뻯ѧ��Ӧ���ݴ˽�ϻ�ѧ����ʽ�ļ�����з�����ɣ�
����⣺��1��ͨ���������ݷ�����֪����һ�μ���20gϡ���ᣬ���������������20g-17.2g=2.8g���ڶ����ټ���20gϡ����ʱ������������ּ�����17.2g-14.4g=2.8g���������ټ���20gϡ����ʱ�����������������14.4g-11.6g=2.8g�����Ĵ��ټ���20gϡ����ʱ��ʣ�������������ٷ����仯��˵��ͭ���Ͻ������Ѿ���Ӧ�꣬ʣ��Ĺ���Ϊͭ��������Ϊ11.6g��
�ʴ�Ϊ��11.6��
��2�����ݣ�1���ķ�����֪����3�κ�Ͻ��е���ȫ��������ȫ���ʴ�Ϊ��3��
��3���ɱ������ݷ�����֪��ÿ�μ���20gϡ��������2.8g��ǡ����ȫ��Ӧ��
��20gϡ������Һ�����ʵ�����Ϊx��
Fe+H2SO4=FeSO4+H2��
56 98
2.8g x
=
��x=4.9g
��20gϡ������Һ�����ʵ�����Ϊ4.9g��
�ʴ�Ϊ��11.6��
��2�����ݣ�1���ķ�����֪����3�κ�Ͻ��е���ȫ��������ȫ���ʴ�Ϊ��3��
��3���ɱ������ݷ�����֪��ÿ�μ���20gϡ��������2.8g��ǡ����ȫ��Ӧ��
��20gϡ������Һ�����ʵ�����Ϊx��
Fe+H2SO4=FeSO4+H2��
56 98
2.8g x
| 56 |
| 98 |
| 2.8g |
| x |
��20gϡ������Һ�����ʵ�����Ϊ4.9g��
�����������ѶȽϴ���Ҫ����ѧ������ͼ�����ݡ���ѧ����ʽ�ļ�����н�����������������ÿ�μ���ϡ����ǰ����������Ϊ��Ӧ���������������жϼ���ϡ����ķ�Ӧ�������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
�����Ŀ