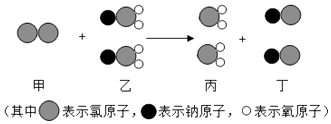
��Ŀ����
����Ŀ����ҵ�����Ĵ����г����������Ȼ������ʣ��ֳ�ȡ12.0g������Ʒ���ձ��У�����ˮʹ����ȫ�ܽ⣬�����Ȼ�����Һ����������ü����Ȼ�����Һ�����������ɳ�����������ϵ��ͼ��ʾ������Ӧ�Ļ�ѧ����ʽ��CaCl2+Na2CO3�TCaCO3��+2NaCl�� 
��1��������Ʒ��̼���Ƶ�����������Ҫ��д��������̣���ȷ��0.1%����
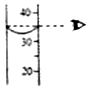
��2����Ҫ�����Ȼ�����Һ�����ʵ�������������ʹ��ͼ��������ʾ���ĵ������ţ�����Ӧ�����ݣ�
���𰸡�
��1���⣺��ͼ���Կ������ɵ�̼��Ƶ�����Ϊ10g
��̼���Ƶ�����Ϊx
CaCl2+ | Na2CO3�T | CaCO3��+2NaCl |
106 | 100 | |
x | 10g |
![]() =
= ![]()
x=10.6g
��Ʒ��̼���Ƶ���������Ϊ ![]() ��100%��88.3%
��100%��88.3%
�������Ȼ�����Һ����������ʱ������50g�Ȼ�����Һ��Ӧ�ij���Ϊ4g��125g�Ȼ�����Һ��Ӧ�ij���Ϊ10g�����Ȼ�����ȫ��Ӧ�����Կ��Ը���a�����b�����ݼ��㣮
��2��a����b
���������⣺��2����Ҫ�����Ȼ�����Һ�����ʵ�������������ʹ��ͼ��������ʾ���ĵ� a����b����Ӧ�����ݣ�
�����㾫�������ڱ��⿼��ĸ��ݻ�ѧ��Ӧ����ʽ�ļ��㣬��Ҫ�˽�����ʼ�������=ϵ������Է�������֮�Ȳ��ܵó���ȷ�𰸣�

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�