��Ŀ����
��10�֣�������ʹ�����Ľ������ϡ�
(1)�����ֶ������Ͻ����к�̼���ϸߵ���_______________��
��2�����Ǵ���ʹ�õ������Ͻ�����Ǵ�����������Ϊ���ĺϽ���и����������ܣ�����ֱȴ���Ӳ�� �����С������
(3) ��ͼ��ʾ��Һ̬������̼���������ش��������⡣

��ͼ�����������������л����ϵ���________________��ֻдһ�֣���
�ڼ�ѹ��С��ƿ��Һ̬������̼������������ԭ����___ ______�� ________��
���ڸ�ƿ����Ϳ�����������____________ _______��
�ܳ���������������Ҫԭ�ϣ�д����CO�ͳ����������Ļ�ѧ����ʽ ��
(4) ��ͼ���й�����ת����ϵ��ʾ��ͼ������A��D��Ϊ̼���Σ�G����õ��ܼ���������ת��û���漰�Ļ�����Ӧ������____________ _______��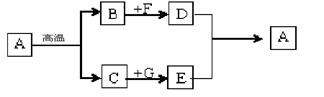
(1)�����ֶ������Ͻ����к�̼���ϸߵ���_______________��
��2�����Ǵ���ʹ�õ������Ͻ�����Ǵ�����������Ϊ���ĺϽ���и����������ܣ�����ֱȴ���Ӳ�� �����С������
(3) ��ͼ��ʾ��Һ̬������̼���������ش��������⡣

��ͼ�����������������л����ϵ���________________��ֻдһ�֣���
�ڼ�ѹ��С��ƿ��Һ̬������̼������������ԭ����___ ______�� ________��
���ڸ�ƿ����Ϳ�����������____________ _______��
�ܳ���������������Ҫԭ�ϣ�д����CO�ͳ����������Ļ�ѧ����ʽ ��
(4) ��ͼ���й�����ת����ϵ��ʾ��ͼ������A��D��Ϊ̼���Σ�G����õ��ܼ���������ת��û���漰�Ļ�����Ӧ������____________ _______��

��10�֣�
(1) ����
(2) ��
��3���� ���� ��
�� ���� �� �������� ��
�� ��ֹ�������� ��
�ܻ�ѧ����ʽ3CO+Fe2O3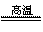 2Fe+3CO2��
2Fe+3CO2��
(4) �û���Ӧ ��
(1) ����
(2) ��
��3���� ���� ��
�� ���� �� �������� ��
�� ��ֹ�������� ��
�ܻ�ѧ����ʽ3CO+Fe2O3
 2Fe+3CO2��
2Fe+3CO2��(4) �û���Ӧ ��
��1�������ĺ�̼��Ϊ2%-4.3%���ֵĺ�̼��С��2%��2���ֱȴ���Ӳ�ȴ�(3)���л����ϰ������ϡ��ϳ����ϳ���ά�Ȣ�Һ̬������̼����Ҫ���մ������ȣ��ʿ������µ����ã�ͬʱ������̼�ܶȱȿ������Ը����ڿ�ȼ����棬�������������ã����ڸ�ƿ����Ϳ������Ը���������ˮ��������������ã�4��AΪ̼�����ҿ��Ը��·ֽ�����뵽��A̼��ƣ���ֽ�������������ƺͶ�����̼��G����õ��ܼ�����֪G��ˮ��DΪ̼���Σ�D������E��Ӧ����A̼��ƣ�������Ƴ�BΪ������̼��CΪ�����ƣ� DΪ̼���ƣ�EΪ�������ƣ�FΪ�������ơ��������ʶ��Ƴ����ˣ��Ϳ���֪��ÿһ������ʲô��Ӧ�ˣ�û�еķ�Ӧ���;����û���Ӧ��

��ϰ��ϵ�д�
�����Ŀ
 ���ȸ���������
���ȸ��������� ������������Ҳ�ﵽ�������ϵ�Ҫ��ϡ����þ�Ͻ����Ͳ�������
������������Ҳ�ﵽ�������ϵ�Ҫ��ϡ����þ�Ͻ����Ͳ�������