��Ŀ����
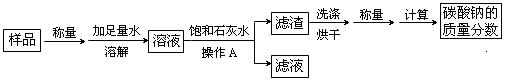
ij̼������Ʒ�к��������Ȼ������ʣ�Ϊ�ⶨ����Ʒ��̼���Ƶ���������������������ʵ�飺
��ش��������⣺
��1������A��������________________���ò����������õ��IJ����������ձ�������� ��
��2����ʵ������м��뱥��ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
��3��Ϊ̽��������Ӧ����Һ�е����ʳɷ֣���ͬѧ����Һ�еμӹ���ϡ���ᣬ���������ݲ�������μ�����ǰ��Һ�е����ʳ��Ȼ������ ���μ�����Ĺ����з�����Ӧ�Ļ�ѧ����ʽΪ ____________��

��ش��������⣺
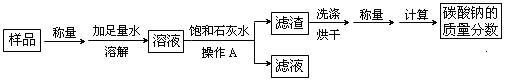
��1������A��������________________���ò����������õ��IJ����������ձ�������� ��
��2����ʵ������м��뱥��ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
��3��Ϊ̽��������Ӧ����Һ�е����ʳɷ֣���ͬѧ����Һ�еμӹ���ϡ���ᣬ���������ݲ�������μ�����ǰ��Һ�е����ʳ��Ȼ������ ���μ�����Ĺ����з�����Ӧ�Ļ�ѧ����ʽΪ ____________��
��1������ ©���������� ��2��Ca(OH)2+Na2CO3=CaCO3��+2NaOH
��3�� Na2CO3��4��Na2CO3+2HCl=2NaCl+H2O+CO2��
��3�� Na2CO3��4��Na2CO3+2HCl=2NaCl+H2O+CO2��
�����������1�����ˣ����ڷ��������Թ�����Һ�壨������Թ��壩�����Բ���A�������ǹ��ˣ��ò����������õ��IJ����������ձ�������У�©����������
��2��̼�����к����Ȼ������ʣ����뱥��ʯ��ˮ��ֻ��̼��������ʯ��ˮ��Ӧ����ѧ����ʽ�ǣ�Ca(OH)2+Na2CO3=CaCO3��+2NaOH
��3����ͬѧ����Һ�еμӹ���ϡ���ᣬ���������ݲ�����˵����Һ�к���̼���μ� Na2CO3��������Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+H2O+CO2��

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ