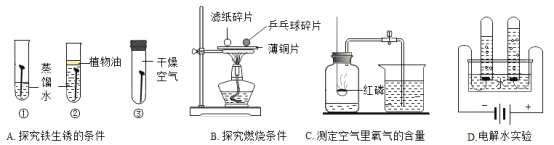
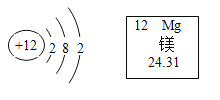
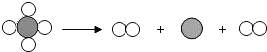��Ŀ����
����Ŀ���Ի��ʵ����ۣ����ҹ涨����ȷ������Ҫ��ijũҵ�Ƽ���Ա��ũ�������ļطʽ����˼�⣬��ȡ4.0g��Ʒ����С�ձ��У�����������ˮ�ܽ������������������Һ��ַ�Ӧ�����������������������������������Һ�����Ĺ�ϵ��ͼ��ʾ�����ٶ����ʲ��μӷ�Ӧ����Ӧ����ʽΪK2SO4+Ba��OH��2�TBaSO4��+2KOH��ͨ������ط�������ص����������жϸò�Ʒ____���ǡ����ϰ�װ˵����
XX�Ƽط�
��Ҫ�ɷ֣�K2SO4����K2SO4������86%��
���أ�50Kg
XX��������˾
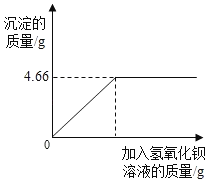
���𰸡��ط�������ص������������ϰ�װ˵��
��������
��40g�����к�����ص�����Ϊx
K2SO4+Ba(OH)2=BaSO4��+2KOH
174 233
x 4.66g
174:233=x:4.66g
x=3.48g
����������ص���������Ϊ3.48g/4.0g��100%=87%��86%
���Լط�������ص������������ϰ�װ˵��

 ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�����Ŀ��ij��ѧ��ȤС��Ϊ̽����������ͭ�Ļ��ǿ������չ�����»��
���������ϣ�
�����ģ��������ڳ�������������е�������Ӧ�������ܵ���������Ĥ���÷�Ӧ�Ļ�ѧ����ʽΪ_____��
���Ա�ʵ�飩
��� | ���� | ���� |
�� | ������δ��ĥ����˿����CuSO4��Һ�� | ���������� |
�� | �������ĥ�����˿����CuSO4��Һ�� | ��˿����������ɫ���� |
�� | ������δ��ĥ����˿����CuCl2��Һ�� | ��˿����������ɫ���� |
��1���Ƚ�ʵ���Һ�ʵ��_____���������������������ɵ�֪����ĥ���ƻ���������Ĥ��
��2��ʵ�����з�Ӧ�Ļ�ѧ����ʽΪ_____���ݴ˿�֪�������Al��Cu_____������ǿ��������������
��3��С��ͬѧ��ʵ�����������з�������ΪH2O����������Ĥ���ƻ����á����˹۵����ϱ�����ͬѧ����������_____��
���²���̽����
С��ͬѧ���ʵ������������ۺ�²⣺Cl-�ƻ�����������Ĥ��
Ϊ����˲²��Ƿ���ȷ��������������֧�Թ��м�����ͬ��CuSO4��Һ�������������δ��ĥ����˿��Ȼ��������µ�̽����
���� | ���� | ���� | ���� |
��1����һ֧�Թ����ټ��� NaCl���� | ��˿������ ����ɫ���� | ��������Ĥ ���ƻ� | Na+���_____�� �ƻ���������Ĥ |
��2������һ֧�Թ����ټ��� Na2SO4���� | ��_____ | ��������Ĥ δ���ƻ� | Na+��SO42������ �ƻ���������Ĥ |
�������뷴˼��
�ó����ۣ�ǰ���²�_____��������ȷ����������ȷ�������ܽᷴ˼������̽����������˱ȽϷ��Ϳ��Ʊ�������