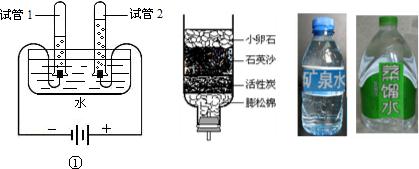
��Ŀ����
�����벻��ˮ������ˮ��Դ��ÿλ�����岻�ݴǵ����Σ�
��1��������Ͽ���ˮ����
��2���ӱ仯�Ͽ���ˮֱͨ����ֽ⣬������
��3������Ⱦ�Ͽ����������������ˮ����Ⱦ����
A����ҵ��ˮֱ���ŷ� B����ҵ�������������ŷ�
C����ֹʹ�ú���ϴ�·� D������ʹ��ũҩ�����������ʳ����
��4���Ӿ����Ͽ�������ˮ���������г�ʹ��
��5������������
��1��������Ͽ���ˮ����
��Ԫ�غ���Ԫ��
��Ԫ�غ���Ԫ��
��ɵģ���2���ӱ仯�Ͽ���ˮֱͨ����ֽ⣬������
��
��
������δ�����������Դ--���������ˮ�õ������������������Ϊ2��1
2��1
����3������Ⱦ�Ͽ����������������ˮ����Ⱦ����
AD
AD
��A����ҵ��ˮֱ���ŷ� B����ҵ�������������ŷ�
C����ֹʹ�ú���ϴ�·� D������ʹ��ũҩ�����������ʳ����
��4���Ӿ����Ͽ�������ˮ���������г�ʹ��
����̿
����̿
����ȥˮ����ζ��ɫ�أ���5�������У�����
����ˮ
����ˮ
�ü�������Ӳˮ����ˮ��Ҫ��ʹӲˮת��Ϊ��ˮ���õķ��������
���
����������1������ˮ�����Ԫ�ط����ش�
��2�����ݵ��ˮ��ʵ������ͽ��۷����ش�
��3������ˮ����ȾԴ������
��4�����ݻ���̿�������Է�����
��5������Ӳˮ����ˮ�����ˮ��Ӧ������ͬ���������г�����еķ�����Ӳˮ������
��2�����ݵ��ˮ��ʵ������ͽ��۷����ش�
��3������ˮ����ȾԴ������
��4�����ݻ���̿�������Է�����
��5������Ӳˮ����ˮ�����ˮ��Ӧ������ͬ���������г�����еķ�����Ӳˮ������
����⣺��1�����ʵ������Ԫ����������������Ͽ���ˮ������Ԫ�غ���Ԫ����ɵģ�
��2��ˮֱͨ����ֽ⣬�ڸ�������δ�����������Դ--�������������������������������������������Ϊ2��1��
��3��A����ҵ��ˮֱ���ŷţ������ˮ����Ⱦ��
B����ҵ�������������ŷţ��������ˮ����Ⱦ��
C����ֹʹ�ú���ϴ�·ۣ��������ˮ����Ⱦ��
D������ʹ��ũҩ�����������ʳ�����������ˮ����Ⱦ��
��4������̿�������ԣ��ھ���ˮʱ����ʹ�û���̿����ȥˮ����ζ��ɫ�أ�
��5�������У����Է���ˮ�ü�������Ӳˮ����ˮ����ˮ�м������ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ�������г�����еķ�����Ӳˮ������
�ʴ�Ϊ����1����Ԫ�غ���Ԫ�أ���2������2��1����3��AD����4������̿����5������ˮ����У�
��2��ˮֱͨ����ֽ⣬�ڸ�������δ�����������Դ--�������������������������������������������Ϊ2��1��
��3��A����ҵ��ˮֱ���ŷţ������ˮ����Ⱦ��
B����ҵ�������������ŷţ��������ˮ����Ⱦ��
C����ֹʹ�ú���ϴ�·ۣ��������ˮ����Ⱦ��
D������ʹ��ũҩ�����������ʳ�����������ˮ����Ⱦ��
��4������̿�������ԣ��ھ���ˮʱ����ʹ�û���̿����ȥˮ����ζ��ɫ�أ�
��5�������У����Է���ˮ�ü�������Ӳˮ����ˮ����ˮ�м������ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ�������г�����еķ�����Ӳˮ������
�ʴ�Ϊ����1����Ԫ�غ���Ԫ�أ���2������2��1����3��AD����4������̿����5������ˮ����У�
������ˮ�Ǵ���Ȼ������������Ҫ�ı�����Դ֮һ������һ��Ҫ�ú���ϧ������ˮ��Դ����Լ��ˮ��

��ϰ��ϵ�д�
 �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д� ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д�
�����Ŀ