��Ŀ����
����ۡ����û�ѧ������ϵ��һ���ǻ�ѧѧ�Ƶ��ص㣮
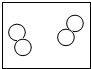
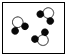
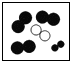
��1��������б��������л��С�������λ����д����Ӧ������
��2���۲�A��B��C��D�ķ�ͼ������Bͼ��ÿ�����Ӻ���
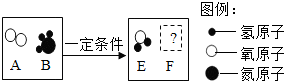
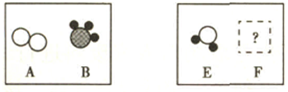
��3����һ���������£�ͼA��B�е������ܹ�������ѧ��Ӧ����E��F������ʾ��ͼ������ʾ��

����FΪ��������������������壬��÷�Ӧ�����ɵ�E��F��������
����FΪ�������A��B�ķ��Ӹ�����Ϊ5��4����÷�Ӧ�Ļ�ѧ����ʽΪ
��1��������б��������л��С�������λ����д����Ӧ������
| A | B | C | D | |
| ��ʾ��ͼ |  |
 |
 |
 |
| ��ѧ���� | 2O2 | 3NH3 | 3H2O | 3N2��O2��H2 3N2��O2��H2 |
4
4
��ԭ�ӣ�����������ʾ�������۹��ɵ���D
D
����ʾ��ͼ����ĸ��ţ�����3����һ���������£�ͼA��B�е������ܹ�������ѧ��Ӧ����E��F������ʾ��ͼ������ʾ��

����FΪ��������������������壬��÷�Ӧ�����ɵ�E��F��������
27��14
27��14
������FΪ�������A��B�ķ��Ӹ�����Ϊ5��4����÷�Ӧ�Ļ�ѧ����ʽΪ
5O2+4NH3
6H2O+4NO
| ||
5O2+4NH3
6H2O+4NO
��
| ||
��������1������ͼƬ��ԭ�ӵ������Լ����ӵĽṹ�������
��2������Bװ���и÷��ӵĽṹ�Լ��������۹����ص������ɣ�
��3������ͼʾ��ԭ�ӵ������Լ������غ㶨�ɵ��й�֪ʶ������ɣ�
��2������Bװ���и÷��ӵĽṹ�Լ��������۹����ص������ɣ�
��3������ͼʾ��ԭ�ӵ������Լ������غ㶨�ɵ��й�֪ʶ������ɣ�
����⣺��1������D�к��е����ַ��ӵ������֪D�к���3���������ӡ�1�������Ӻ�1���������ӣ�
��2����ͼB�еķ��ӽṹ��֪�÷����к���4��ԭ�ӣ�����ﺬ�ж��ַ��ӣ������ڻ�������D��
��3������F�ǵ���ʱ�䷴Ӧ���������백����������ˮ�뵪�����䷽��ʽ��3O2+4NH3
6H2O+2N2����ˮ�뵪����������=��6��18������4��14��=27��14��
�����������غ㶨�ɿ�֪Fһ���ǵ����������AB�ķ��Ӹ�����5��4������д������ʽ��5O2+4NH3
6H2O+4NO��
�ʴ�Ϊ����1��3N2��O2��H2����2��4��D����3��27��14��5O2+4NH3
6H2O+4NO��
��2����ͼB�еķ��ӽṹ��֪�÷����к���4��ԭ�ӣ�����ﺬ�ж��ַ��ӣ������ڻ�������D��
��3������F�ǵ���ʱ�䷴Ӧ���������백����������ˮ�뵪�����䷽��ʽ��3O2+4NH3
| ||
�����������غ㶨�ɿ�֪Fһ���ǵ����������AB�ķ��Ӹ�����5��4������д������ʽ��5O2+4NH3
| ||
�ʴ�Ϊ����1��3N2��O2��H2����2��4��D����3��27��14��5O2+4NH3
| ||
������������ԭ�ӹ��ɣ�ͬ��ԭ�ӹ��ɵķ���Ϊ���ʷ��ӣ���ͬ��ԭ�ӹ��ɵķ���Ϊ��������ӣ���������ֻ��һ�ַ��ӣ�����ﺬ�ж��ַ��ӣ�

��ϰ��ϵ�д�
 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
�����Ŀ
����ۡ����û�ѧ������ϵ��һ���ǻ�ѧѧ�Ƶ��ص㣮
��1��������б��������л��С�������λ����д����Ӧ������
��2���۲�A��B��C��D�ķ�ͼ������Bͼ��ÿ�����Ӻ��� ��ԭ�ӣ�����������ʾ�������۹��ɵ��� ����ʾ��ͼ����ĸ��ţ���
��3����һ���������£�ͼA��B�е������ܹ�������ѧ��Ӧ����E��F������ʾ��ͼ������ʾ��

����FΪ��������������������壬��÷�Ӧ�����ɵ�E��F�������� ��
����FΪ�������A��B�ķ��Ӹ�����Ϊ5��4����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��1��������б��������л��С�������λ����д����Ӧ������
| A | B | C | D | |
| ��ʾ��ͼ | 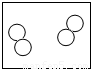 |  | 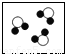 |  |
| ��ѧ���� | 2O2 | 3NH3 | 3H2O |
��3����һ���������£�ͼA��B�е������ܹ�������ѧ��Ӧ����E��F������ʾ��ͼ������ʾ��

����FΪ��������������������壬��÷�Ӧ�����ɵ�E��F�������� ��
����FΪ�������A��B�ķ��Ӹ�����Ϊ5��4����÷�Ӧ�Ļ�ѧ����ʽΪ ��
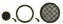 ����С��ֱ������ԭ�ӡ���ԭ�Ӻ͵�ԭ�ӣ�
����С��ֱ������ԭ�ӡ���ԭ�Ӻ͵�ԭ�ӣ�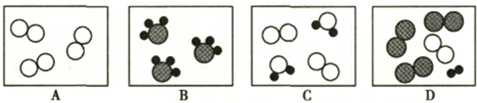
 ��������
��������