��Ŀ����
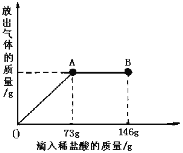
����Ŀ����6�֣���֪ Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl ��ɵĹ�������������μ������ʷ���Ϊ10����ϡ���ᡣ�ų��������������������ϡ�����������ϵ������ͼ��ʾ��
���������ش����⣺
��1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH 7���������������
��2��ԭ�������̼���Ƶ�������
��3�����μ�ϡ������ͼ��A��ʱ���ձ���Ϊ��������Һ��ͨ����������������ʵ�����������������������һλС����
���𰸡���1���� ��2��10.6g��3��24.2%
��������
�����������ͼ��֪����A��ʱ̼������ϡ����ǡ����ȫ��Ӧ����B��ʱ�����������Һ��PH<7��
�⣺73g10%��ϡ�����к�HCl�������ǣ�73g��10%=7.3g
��μӷ�Ӧ��̼���Ƶ�����Ϊx����Ӧ���ɵ��Ȼ��Ƶ�����Ϊy�����ɵĶ�����̼������Ϊz��
Na2CO3 +2HCl===2NaCl +CO2�� +H2O
106 73 117 44
x 7.3g y z
![]() x=10.6g
x=10.6g
![]() y=11.7g
y=11.7g
![]() z=4.4g
z=4.4g
�ձ��ﲻ������Һ���ʵ�����Ϊ:11.7g+(20.4g-10.6g)=21.5g
�ձ��ﲻ������Һ������Ϊ��20.4g+73g-4.4g=89g
�ձ��ﲻ������Һ�����ʵ���������Ϊ��![]() ��100%=24.2%
��100%=24.2%

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�����Ŀ����5�֣�ij�о�С����ѧϰ�����Ļ�ѧ����ʱ���֣���˿ȼ��û�л��棬����ȼ��ȴ�������Ļ��档��С��ͬѧ����������̽����
��1��̽��һ������ȼ�ղ��������ԭ����ʲô��
��ȼ������������һ���������棬�����������ʣ�����һ���ܿڵ�ȼ��Ҳ�л����������ͼ��ʾ����
![]()
�ɴ˿�֪������ȼ�ղ����Ļ������� �����̬������̬��������ȼ���γɵġ�д������ȼ�յ����ֱ���ʽ
��2��̽����������ȼ�ղ�������ĸ���ԭ����ʲô��
���������ϡ�
���� | �۵��u�� | �е��u�� | ȼ��ʱ�¶��u�� |
ʯ�� | 50~70 | 300~550 | Լ600 |
�� | 1535 | 2750 | Լ1800 |
�� | 97.8 | 883 | Լ1400 |
���ϱ���֪������ȼ���ܷ������������ ����۵㡱�е㡱����ȼ��ʱ�¶��йء��ɴ��Ʋ⣺����ȼ��ʱ�� ����С���û�С������������
��3��ľ̿��������ȼ��û�л��棬��������ľ̿ȼ��ʱ��������棬��ԭ�������