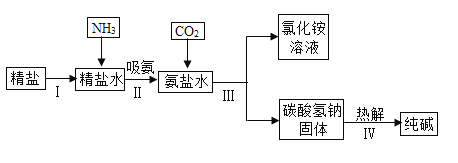
��Ŀ����
����Ŀ�������������ϣ���ȼ���ǹ��ϵĵ�������δ���������������Դ����������ʯ�͡�ú����Ȼ���ȴ�ͳ��ʯȼ�ϣ��ҹ���ҵ������ȼ������2030�ꡣ��ش�
��1���ڿ�ȼ���У�ƽ��ÿ46��ˮ���ӹ���8������ÿ����������һ��������ӻ�һ�������ˮ���ӣ���ÿ8��������6�������˼�����ӣ�����2����2�������ˮ��������䣬���ȼ����ƽ����������и����е���_____��
ACH4��2H2O BCH4��6H2O CCH4��8H2O DCH4��10H2O
��2����ȼ������Ҫ�ɷ�Ϊ����ˮ��������1�������ȼ�����ɴ���100��200����ļ������壬�������̼����Ԫ��֮�����������Ϊ___����Ȼ��ȼ��ʱ����___ɫ���棬��д����Ȼ����ȫȼ��ʱ�Ļ�ѧ����ʽΪ_____________��
��3����ȼ������Ϊ����ʮһ���������Դ���������������������ȼ�����ŵ��У�Ҫ��˵���������㣩______________����Ŀǰ�����ڿ��ɼ����ϻ��������ѣ���������м�������һ��й¶���ܵ��µĺ����_______________��
��4�����������֣��ҹ��Ϻ������̺��ḻ�Ŀ�ȼ����Ϊ�ҹ���ʹ�ø�Ч������Դ������ʮ�ֹ����Ŀ���ǰ��������Ϊ�ܿ������õ�����Դ���У�������д���֣�__________��
���𰸡�C 3:1 �� CH4+2O2 ![]() CO2+ 2H2O �����Ի����Ѻá�������Ϊ��̬�����䣬��ʱ��Һ���� ��������ӷ��������У������ȫ������ЧӦ ̫���ܡ����ܡ������ܡ���ϫ�ܵ�
CO2+ 2H2O �����Ի����Ѻá�������Ϊ��̬�����䣬��ʱ��Һ���� ��������ӷ��������У������ȫ������ЧӦ ̫���ܡ����ܡ������ܡ���ϫ�ܵ�
��������
��1���ڿ�ȼ���У�ƽ��ÿ46��ˮ���ӹ���8������ÿ����������һ��������ӻ�һ�������ˮ���ӣ���ÿ8��������6�������˼�����ӣ�����2����2�������ˮ��������䣬��ÿ�ȼ���к���46+2=48������ˮ���ӣ���6��������ӣ�����������ˮ���ӵı�Ϊ6��48=1:8�����ȼ����ƽ����ɣ�CH4��8H2O��
��ѡ��C��
��2��������̼����Ԫ��֮�����������Ϊ12����1��4��=3:1����Ȼ��ȼ��ʱ������ɫ���棬��Ȼ����ȫȼ��ʱ����ˮ�Ͷ�����̼�Ļ�ѧ����ʽΪ�� CH4+2O2 ![]() CO2+ 2H2O��
CO2+ 2H2O��
��3����ȼ������Ϊ����ʮһ���������Դ������ȼ�����ŵ��У������Ի����Ѻá�������Ϊ��̬�����䣬��ʱ��Һ���ȡ���Ŀǰ�����ڿ��ɼ����ϻ��������ѣ���������м�������һ��й¶���ܵ��µĺ���ǣ���������ӷ��������У������ȫ������ЧӦ��
��4���ܿ������õ�����Դ���У�̫���ܡ����ܡ������ܡ���ϫ�ܵȡ�

����Ŀ���ղ�������ֹ��߾���ⷢ�֣�������ϲ�����������̽��������������ұ���ijʵ��С������̽���������Ľ��������Ϊ����̽����Ŀ��
��ʵ�鷽��������ͬ�¶��£�ȡ��С��ͬ��������������ֽ�����Ƭ���ֱ�Ͷ����������������������������ϡ�����У���Ӧ��Mn��+2�ۣ���
���۲�����ͨ���۲�ʵ��������д�±���
���� | �� | �� |
�����ᷴӦ���� | �ų��������ʻ��� | �ų��������ʽϿ� |
���� | �����_____�������������������� | |
�����۹��ɣ�
��1�����˱ȽϽ����ֱ����ᷴӦ�����������ʵĿ����������پٳ�һ�ֿɱȽ�����ֽ������ǿ���ķ�������������Ƶ�ʵ�鷽����_________��
��2��д���������������е�һ�����ʷ�����Ӧ�Ļ�ѧ����ʽΪ_______����Ӧ�Ļ�������Ϊ_________��