��Ŀ����
��47����һ�ַǽ������ʺ�һ����������ɵĺ�ɫ������������������ȣ���ַ�Ӧ�Ƶ�ʣ����������Ϊ36�ˣ��ù���Ҳ����һ�ֵ��ʺ�һ����������ɣ����ʣ��������100��ϡ���ᣬ���е�������������ǡ����ȫ��Ӧ�����˺����������ʺ�ɫ���ش�
��1��ԭ�������ʲô������ɣ�
��2��ԭ������к����ʶ��ٿˣ�
��3�����˺����õ���Һ���������ٿ�ˮ�������γ�t��ʱ�ı�����Һ����t��ʱ������Һ�����ʵ��ܽ��Ϊ16�ˣ�
�⣺��1����Ϊ��ɫ��������ᷴӦ������ɫ���˺�ɫ������Ϊ����ͭ������һ�ֺ�ɫ���ʷ�Ӧ���ɳʹ�����ɫ������ȷ���ú�ɫ������̼��ԭ�������C��CuO��ɣ��ʴ�Ϊ��C��CuO��
��2����3����ԭ��ȫ���е�������ΪX������CuO����ΪY
C+2CuO 2Cu+CO2��
2Cu+CO2��
12 160 44
X Y 47g-36g
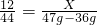
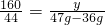
X=3g
Y=40g
��ԭ������е���Ϊ3g
��3��������֪����ͭ������Ϊ47g-40g-3g=4g
������CuSO4����ΪZ��
CuO+H2SO4�TCuSO4+H2O
80 160
4g Z

Z=8g
����������ˮm���ܴﵽ��
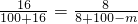
m=50g
����Һ������50��ˮ�������γ�t��ʱ�ı�����Һ��
��������1����Ϊ��ɫ��������ᷴӦ������ɫ���˺�ɫ������Ϊ����ͭ������һ�ֺ�ɫ���ʷ�Ӧ���ɳʹ�����ɫ������ȷ���ú�ɫ������̼��
��2�����������غ㶨��������ɶ�����̼���������Ӷ����ԭ������к��е��ʵ�������
��3����ɫ���������ᷴӦ������ɫ��Һ�����ˣ�������Һ�����ʣ�
���������⿼�黯ѧ����ʽ����ؼ��㣬�ۺ���ǿ������ϸ�������
��2����3����ԭ��ȫ���е�������ΪX������CuO����ΪY
C+2CuO
 2Cu+CO2��
2Cu+CO2��12 160 44
X Y 47g-36g
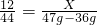
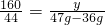
X=3g
Y=40g
��ԭ������е���Ϊ3g
��3��������֪����ͭ������Ϊ47g-40g-3g=4g
������CuSO4����ΪZ��
CuO+H2SO4�TCuSO4+H2O
80 160
4g Z

Z=8g
����������ˮm���ܴﵽ��
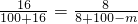
m=50g
����Һ������50��ˮ�������γ�t��ʱ�ı�����Һ��
��������1����Ϊ��ɫ��������ᷴӦ������ɫ���˺�ɫ������Ϊ����ͭ������һ�ֺ�ɫ���ʷ�Ӧ���ɳʹ�����ɫ������ȷ���ú�ɫ������̼��
��2�����������غ㶨��������ɶ�����̼���������Ӷ����ԭ������к��е��ʵ�������
��3����ɫ���������ᷴӦ������ɫ��Һ�����ˣ�������Һ�����ʣ�
���������⿼�黯ѧ����ʽ����ؼ��㣬�ۺ���ǿ������ϸ�������

��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ