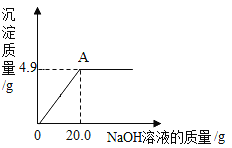
��Ŀ����
����Ŀ��С�մ����Ҫ�ɷ���̼�����ƣ��������������������Ȼ��ƣ���ѧ��ȤС���ͬѧҪͨ��ʵ�����ⶨijƷ��С�մ���Ʒ��̼�����Ƶ�����������
(1)ȷ��ȡ9.0gС�մ���Ʒ�����ձ��У���μ�����������Ϊ5%��ϡ������ǡ�ò��ٲ�������Ϊֹ��������ϡ����73.0g���ձ���û�в��������������Ʒ��̼�����Ƶ�����������(д���������)____
(2)�������һ��������ʵ��ԭ���Ͳ�������������ͬ��ʵ�飬�ⶨС�մ���Ʒ��̼�����Ƶ���������_______��
���𰸡�93.3%ȷ��ȡngС�մ���Ʒ�����Թ��У����������ٲ�������Ϊֹ��ȷ����ʣ����壨Na2CO3������Ϊmg�����÷���ʽ�ɼ�����Ʒ��̼�����Ƶ������������������������������ɣ�
��������
��̼�����Ƶ�����Ϊx
NaHCO3 �� HCl=== NaCl��H2O��CO2��
84���� 36.5
x������ 73.0g��5%
![]() =
=![]()
x��8.4 g
��Ʒ��̼���Ƶ���������Ϊ![]() ��100%��93.3%
��100%��93.3%
����Ʒ��̼�����Ƶ���������Ϊ93.3%��
��2��ȷ��ȡngС�մ���Ʒ�����Թ��У����������ٲ�������Ϊֹ��ȷ����ʣ����壨Na2CO3������Ϊmg�����÷���ʽ�ɼ�����Ʒ��̼�����Ƶ������������������������������ɣ�
����Ʒ��̼�����Ƶ�����Ϊx
2NaHCO3![]() Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
168 18+44
x n-m
![]() =
=![]()
x=![]() ����n-m��
����n-m��
С�մ����������Ϊ![]() ����n-m����n��100%
����n-m����n��100%

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ľ��(��Ҫ�ɷ�ΪK2CO3�����ʲ�����ˮ)��Ʒ�����ܻ�������K2SO4��KCl��ij��ѧ��ȤС�����������ʵ�飺
(1)��Ʒ��Ԥ������

�ٲ���a������Ϊ__________________����Ŀ����____________________
����ҺA����Ҫ�������ӣ���CO32-�⣬���ܻ�����_______________(�����ӷ���)
(2)��Ʒ�ɷֵ�ȷ�������ʵ�鷽�������������������ѡ�Լ���ϡ���ᡢϡ���ᡢϡ���ᡢ�Ȼ�����Һ�����ᱵ��Һ����������Һ
ʵ����� | Ԥ������ͱ�Ҫ���� |
����1��ȡ��ҺA�������Թ��У��������� _______________����������ã����ˡ� |
|
����2��ȡ��������1���õ���Һ���Թ��У�____________ | ______��˵����Ʒ��δ����KCl |
����3��ȡ��������1���õ��������Թ��У�__________________ | ______��˵����Ʒ��������K2SO4 |