��Ŀ����
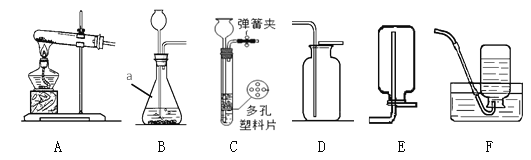
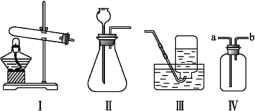
����Ŀ����һ�ο�����,С��ͬѧ����ȡij������,��ѧ��ʦΪ���ṩ����ͼ��ʾ��������ҩƷ:
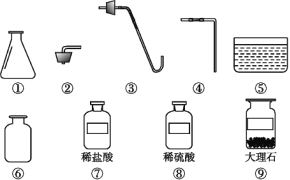
(1)����ΪС��ͬѧѡ����ȡ����������Ҫ��������ҩƷ(����):___________________��
(2)��ȡ������ʱ������Ӧ�Ļ�ѧ����ʽΪ________________________________��
(3)����������ļ���ƿ,Ӧ��_______(����������������)����������,������______________________��
(4)��ȡ�������װ�û�����ȡ________,��д����ȡ��������Ļ�ѧ����ʽ:_______________________��
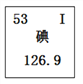
���𰸡� �٢ڢܢޢߢ� CaCO3+2HCl=CaCl2+H2O+CO2�� �� ������̼���ܶȱȿ����Ĵ� ���� 2H2O2![]() 2H2O+O2��
2H2O+O2��
��������(1)��Ϊ�д���ʯ��ϡ���ᣬ�����ȡ�������Ƕ�����̼��ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ����õ���������ҩƷ�У���ƿ��������Ƥ�������ܡ�����ƿ��ϡ���ᡢ����ʯ��(2)��̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����(3)����������̼�ļ���ƿ��Ӧ�������������ϣ������Ƕ�����̼���ܶȱȿ������ܶȴ�(4)�����˫��ˮ�Ͷ��������������Ͳ���Ҫ���ȣ����������ڶ�������������������������ˮ����������ѧ����ʽΪ��2H2O2![]() 2H2O+O2����
2H2O+O2����

 ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�