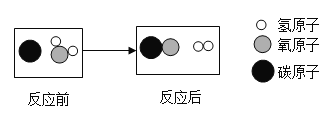
��Ŀ����
����Ŀ��С���Լ���Ļ�ͭ(ͭп�Ͻ�)Ϊ�������Ƶ���(����ͭƷ��)���Ʊ��������£�
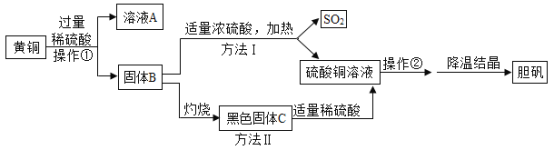
(1)�����ٵõ��Ĺ���B����Ҫϴ�ӣ�ϴ�ӵ�������______��
(2)��ҺA��������_______�����չ���B�Ļ�ѧ����ʽΪ_______��
(3)��ɫ����C��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ_______��
(4)�Ʊ������в����ڵ�������_____�����иò���ʱ��ʹ�õ�����������������_____����������ͭ�ᾧ�ķ��������Ƴ�����ͭ���ܽ�����¶ȵı仯Ӱ��______(ѡ��ϴ���)��
(5)���ַ����Ƚϣ��������������ɫ��ѧ��Ҫ��������_______��
���𰸡���ȥ�����ϸ��ŵ�����п������ ����п������ ![]() CuO+H2SO4=CuSO4+H2O ����Ũ�� ��ֹҺ�����Ȳ���������Һ�ηɽ� �ϴ� �����������Ⱦ�����Ķ�������
CuO+H2SO4=CuSO4+H2O ����Ũ�� ��ֹҺ�����Ȳ���������Һ�ηɽ� �ϴ� �����������Ⱦ�����Ķ�������
��������
����ϡ����ѻ�ͭ�е�п�ܽ⣬�õ�����ͭ���ٰ�ͭת�������ͭ���塣
��1��ͨ�����˲����õ��Ĺ���ͭ��ͭ�ı��滹����������п��Һ�����ᣬ����Ҫϴ�ӣ�ϴ�ӵ������dz�ȥ������渽�ŵ�����п�����ᡣ
��2����ҺA�����������ɵ�����п�������������ͭ�Ļ�ѧ����ʽΪ![]() ��
��
��3������ͭ��ϡ���ᷴӦ��������ͭ��ˮ����ѧ����ʽΪCuO+H2SO4=CuSO4+H2O��
��4���ڲ����ں���еIJ�������ȴ�ᾧ�����Բ������ǰ���Һ����ȵı�����Һ���Ǽ���Ũ�����ò���ʹ�õ��������������ǽ��裬��ֹҺ�����Ȳ���������Һ�ηɽ�������ͭ�ᾧ�ķ�����ȴ�ᾧ����֪����ͭ���ܽ�����¶ȵı仯Ӱ��ϴ�
(5)���ַ����Ƚϣ�����Iͭ��Ũ�������ȷ�Ӧ���ɶ��������ж����壬��Ⱦ����������II�����������Ⱦ�����Ķ�������