ĢāÄæÄŚČŻ
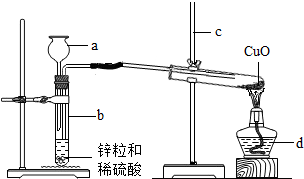
(8·Ö)¶žŃõ»ÆĢ¼µÄ”°²¶×½”±Óė”°·ā“ę”±ŹĒŹµĻÖĪĀŹŅĘųĢå¼õÅŵÄÖŲŅŖĶ¾¾¶Ö®Ņ»”£Źµ¼ŹÉś²śÖŠ£¬¾³£ĄūÓĆ×ćĮæNaOHČÜŅŗĄ“”°²¶×½”±CO2£¬Į÷³ĢĶ¼ČēĻĀ(²æ·ÖĢõ¼ž¼°ĪļÖŹĪ“±ź³ö)”£

¢ŁĶ¼ÖŠ±ź³öµÄĪļÖŹÖŠ£¬ŹōÓŚ¼īĄąĪļÖŹµÄ»ÆѧŹ½ĪŖ £»
¢ŚøĆĮ÷³ĢĶ¼Ėł±ź³öµÄĪļÖŹÖŠ£¬ČÜÓŚĖ®·Å³ö“óĮæČȵÄŃõ»ÆĪļŹĒ £¬ÓėĖ®·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
¢Ū”°²¶×½ŹŅ”±ÖŠ·¢ÉśµÄµÄ»Æѧ·½³ĢŹ½ĪŖ £¬Õūøö”°²¶×½”±¹ż³ĢÖŠ£¬æÉŃ»·Ź¹ÓƵÄĪļÖŹŹĒ ŗĶ ”£

¢ŁĶ¼ÖŠ±ź³öµÄĪļÖŹÖŠ£¬ŹōÓŚ¼īĄąĪļÖŹµÄ»ÆѧŹ½ĪŖ £»
¢ŚøĆĮ÷³ĢĶ¼Ėł±ź³öµÄĪļÖŹÖŠ£¬ČÜÓŚĖ®·Å³ö“óĮæČȵÄŃõ»ÆĪļŹĒ £¬ÓėĖ®·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
¢Ū”°²¶×½ŹŅ”±ÖŠ·¢ÉśµÄµÄ»Æѧ·½³ĢŹ½ĪŖ £¬Õūøö”°²¶×½”±¹ż³ĢÖŠ£¬æÉŃ»·Ź¹ÓƵÄĪļÖŹŹĒ ŗĶ ”£
¢Ł NaOH ¢ŚCaO CaO + H2O=Ca(OH)2””””””
¢ŪCO2 +2 NaOH=Na2CO3 + H2O NaOH”¢CaO
¢ŪCO2 +2 NaOH=Na2CO3 + H2O NaOH”¢CaO
ŹŌĢā·ÖĪö£ŗ¢Ł“æ¼īŹĒĢ¼ĖįÄĘ£¬ŗĶĢ¼ĖįøĘŅ»Ńł¶¼ŹĒŹōÓŚŃĪ£¬Ńõ»ÆøĘŗĶ¶žŃõ»ÆĢ¼¶¼ŹĒŃõ»ÆĪļ£¬ŹōÓŚ¼īµÄŹĒĒāŃõ»ÆÄĘ£¬»ÆѧŹ½ĪŖ£ŗNaOH
¢ŚŃõ»ÆøĘČÜÓŚĖ®£¬»įÓėĖ®·“Ó¦£¬²¢ĒŅ·Å³ö“óĮæµÄČČ£¬»Æѧ·½³ĢŹ½ĪŖ£ŗCaO + H2O=Ca(OH)2””
¢Ū”°²¶×½ŹŅ”±ÖŠĶØČė¶žŃõ»ÆĢ¼½ųČ„ŗó£¬µĆµ½“æ¼īČÜŅŗ£¬ĖłŅŌ·¢ÉśµÄµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗCO2 +2 NaOH=Na2CO3 + H2O£¬æÉŃ»·Ź¹ÓƵÄĪļÖŹæÉŅŌÖ±½Ó“ÓĮ÷³ĢĶ¼ÖŠæÉŅŌ擳ö£¬NaOHŗĶCaO¼Č×÷ĪŖ·“Ó¦£¬ÓÖ×÷ĪŖÉś³ÉĪļ£¬ĖłŅŌæÉŅŌŃ»·Ź¹ÓĆ

Į·Ļ°²įĻµĮŠ“š°ø
 ĆūŹ¦µ¼ŗ½µ„ŌŖĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
ĆūŹ¦µ¼ŗ½µ„ŌŖĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø ĆūŠ£Ćū¾ķµ„ŌŖĶ¬²½ŃµĮ·²āŹŌĢāĻµĮŠ“š°ø
ĆūŠ£Ćū¾ķµ„ŌŖĶ¬²½ŃµĮ·²āŹŌĢāĻµĮŠ“š°ø
Ļą¹ŲĢāÄæ