��Ŀ����
������С��װ�����������ɷ�ΪFe�ۡ�����̿������NaCl��ˮ��ʹ��һ��ʱ������е�Fe�ۻ�ת���Fe2O3�����ʣ�ij��ѧ��ȤС����̽��ʹ�ù��������ơ����������ı��ʳ̶ȣ��ѱ��ʵ�Fe��ռ����ǰFe�۵���������������Ʋ���������̽�����̣����裨1��ȡʳƷ��װ���е������ơ���������һ����������Ĺ�������ˮ�����ˡ�ϴ�ӡ�����������
���裨2��ȡ���裨1���е�����8.0g������������ϡH2SO4��������ַ�Ӧ�����ˡ�ϴ�ӡ�����ù���1.2g��
���裨3��ȡ���裨2���е���Һ������������NaOH��Һ���õ��Ĺ��徭ϴ�Ӻ�ת�Ƶ������У���ּ��ȡ���ȴ���������õ�8.0g Fe2O3��ע����Һ�е�FeԪ����ȫ��ת��ΪFe2O3����
��1��8.0g������Fe��Fe2O3�������ʵ���������
��2���á�����������δ����ʱ��Fe�ۺͻ���̿������֮�ȣ�
��3���á����������ı��ʳ̶ȣ�
���𰸡���������1������̿������ˮ��Ҳ�����ᷴӦ���ʲ��裨2���õ�����1.2g�ǻ���̿����������������-����̿������=������Fe��Fe2O3�������ʵ���������
��2�����ݡ�8.0g×Fe2O3��FeԪ�ص����������������FeԪ�ص�������Ȼ�������̿�������Ƚϼ��ɣ�
��3�����ݡ�������Fe��Fe2O3�������ʵ�������-FeԪ�ص���������������������ʶ����ӵ���Ԫ�ص��������ٳ���Fe2O3����Ԫ������Ԫ�ص������ȣ��Ϳɼ������������������Fe��ռ����ǰFe�۵���������= ×100%�����������ı��ʳ̶ȣ�
×100%�����������ı��ʳ̶ȣ�
����⣺��1���������֪��1.2g�Ĺ����ǻ���̿��������
��8.0g������Fe��Fe2O3�������ʵ�������Ϊ8.0g-1.2g=6.8g��
��2��FeԪ�ص�����Ϊ��8.0g×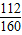 =5.6g��
=5.6g��
��Fe�ۺͻ���̿������֮��Ϊ5.6g��1.2g=14��3��
��3�����������ʶ����ӵ���Ԫ�ص�����Ϊ��6.8g-5.6g=1.2g��
������������Ϊ��1.2g÷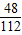 =2.8g��
=2.8g��
�������ı��ʳ̶�=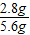 ×100%=50%��
×100%=50%��
�𣺣�1��8.0g������Fe��Fe2O3�������ʵ�������Ϊ6.8g��
��2���á�����������δ����ʱ��Fe�ۺͻ���̿������֮��14��3��
��3���á����������ı��ʳ̶�Ϊ50%��
������������Ҫ����ѧ�����û�ѧʽ���м��������������Ĺؼ�����ȷ����������ϵ��
��2�����ݡ�8.0g×Fe2O3��FeԪ�ص����������������FeԪ�ص�������Ȼ�������̿�������Ƚϼ��ɣ�
��3�����ݡ�������Fe��Fe2O3�������ʵ�������-FeԪ�ص���������������������ʶ����ӵ���Ԫ�ص��������ٳ���Fe2O3����Ԫ������Ԫ�ص������ȣ��Ϳɼ������������������Fe��ռ����ǰFe�۵���������=
 ×100%�����������ı��ʳ̶ȣ�
×100%�����������ı��ʳ̶ȣ�����⣺��1���������֪��1.2g�Ĺ����ǻ���̿��������
��8.0g������Fe��Fe2O3�������ʵ�������Ϊ8.0g-1.2g=6.8g��
��2��FeԪ�ص�����Ϊ��8.0g×
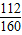 =5.6g��
=5.6g����Fe�ۺͻ���̿������֮��Ϊ5.6g��1.2g=14��3��
��3�����������ʶ����ӵ���Ԫ�ص�����Ϊ��6.8g-5.6g=1.2g��
������������Ϊ��1.2g÷
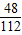 =2.8g��
=2.8g���������ı��ʳ̶�=
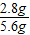 ×100%=50%��
×100%=50%���𣺣�1��8.0g������Fe��Fe2O3�������ʵ�������Ϊ6.8g��
��2���á�����������δ����ʱ��Fe�ۺͻ���̿������֮��14��3��
��3���á����������ı��ʳ̶�Ϊ50%��
������������Ҫ����ѧ�����û�ѧʽ���м��������������Ĺؼ�����ȷ����������ϵ��

��ϰ��ϵ�д�
�����Ŀ
������С��װ�����������ɷ�ΪFe�ۡ�����̿������NaCl��ˮ��ʹ��һ��ʱ������е�Fe�ۻ�ת���Fe2O3�����ʣ�ij��ѧ��ȤС����̽��ʹ�ù��������ơ����������ı��ʳ̶ȣ��ѱ��ʵ�Fe��ռ����ǰFe�۵���������������Ʋ���������̽�����̣�
[�ռ�����]��1��Fe��OH��2�ڿ����п�������Fe��OH��3��
��2�������Լ����Ȳ��ȶ�����������Ӧ�Ľ��������
[ʵ��̽��]
[���ݴ���]����㣺�á����������ı��ʳ̶� ���ޣ���
[�ռ�����]��1��Fe��OH��2�ڿ����п�������Fe��OH��3��
��2�������Լ����Ȳ��ȶ�����������Ӧ�Ľ��������
[ʵ��̽��]
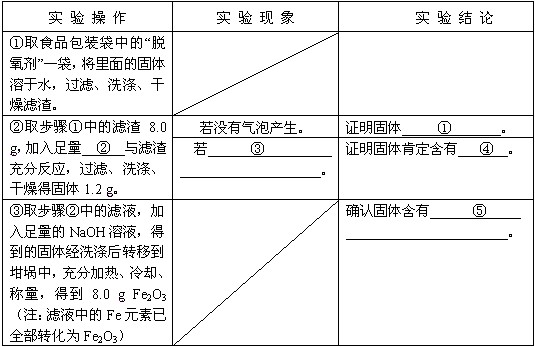
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ��ȡʳƷ��װ���еġ���������һ����������Ĺ�������ˮ�����ˡ�ϴ�ӡ����������� | �� | �� |
| ��ȡ������е�����8.0 g������������ |
��û�����ݲ����� | ֤������� |
| ���� |
֤������϶����Т� | |
| ��ȡ������е���Һ������������NaOH��Һ���õ��Ĺ��徭ϴ�Ӻ�ת�Ƶ������У���ּ��ȡ���ȴ���������õ�8.0 g Fe2O3��ע����Һ�е�FeԪ����ȫ��ת��ΪFe2O3�� | �� | ȷ�Ϲ��庬�Т� |
������С��װ�����������ɷ�ΪFe�ۡ�����̿������NaCl��ˮ��ʹ��һ��ʱ������е�Fe�ۻ�ת���Fe2O3�����ʣ�ij��ѧ��ȤС����̽��ʹ�ù��������ơ����������ı��ʳ̶ȣ��ѱ��ʵ�Fe��ռ����ǰFe�۵���������������Ʋ���������̽�����̣�
[�ռ�����]��1��Fe��OH��2�ڿ����п�������Fe��OH��3��
��2�������Լ����Ȳ��ȶ�����������Ӧ�Ľ��������
[ʵ��̽��]
[���ݴ���]����㣺�á����������ı��ʳ̶� ���ޣ���
[�ռ�����]��1��Fe��OH��2�ڿ����п�������Fe��OH��3��
��2�������Լ����Ȳ��ȶ�����������Ӧ�Ľ��������
[ʵ��̽��]
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ��ȡʳƷ��װ���еġ���������һ����������Ĺ�������ˮ�����ˡ�ϴ�ӡ����������� | �� | �� |
| ��ȡ������е�����8.0 g������������______��������ַ�Ӧ�����ˡ�ϴ�ӡ�����ù���1.2 g�� | ��û�����ݲ����� | ֤�������______�� |
| ����______�� | ֤������϶����Т�______�� | |
| ��ȡ������е���Һ������������NaOH��Һ���õ��Ĺ��徭ϴ�Ӻ�ת�Ƶ������У���ּ��ȡ���ȴ���������õ�8.0 g Fe2O3��ע����Һ�е�FeԪ����ȫ��ת��ΪFe2O3�� | �� | ȷ�Ϲ��庬�Т�______�� |