��Ŀ����
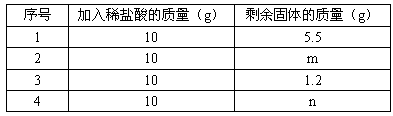
��3�֣�ijͬѧȡ�ؿ�ʯ��Ʒ��������Ʒ�е�̼��Ƶ������������м�⣬�����ǣ�ȡ��ʯ��Ʒ8g����40g������Һ���Ĵμ��룬�����������±�����֪ʯ��ʯ��Ʒ�е����ʲ�����ˮ���������ᷴӦ������㣺| ��� | ����ϡ�����������g�� | ʣ������������g�� |
| 1 | 10 | 5.5 |
| 2 | 10 | m |
| 3 | 10 | 1.2 |
| | | n |
��1���ϱ���m����ֵ ��
��2����Ʒ��̼��Ƶ�����������
��3��Ҫ��ȡ4.4g������̼���躬̼�����������Ϊ80%��ʯ��ʯ���ٿˣ�
��2��CaCO3����
 100�G��85��
100�G��85�� CaCO3 + 2HCl �� CaCl2 + H2O + CO2��
100 44
X 4.4g
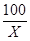 ��
�� 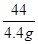
X��10g
ʯ��ʯ������Ϊ:10g / 80%��12.5g����:
��

ijͬѧȡ�ؿ�ʯ��Ʒ��������Ʒ�е�̼��Ƶ������������м�⣬�����ǣ�ȡ��ʯ��Ʒ8g����40g������Һ���Ĵμ��룬�����������±�����֪ʯ��ʯ��Ʒ�е����ʲ�����ˮ���������ᷴӦ������㣺
| ��� | ����ϡ�����������g�� | ʣ������������g�� |
| 1 | 10 | 5.5 |
| 2 | 10 | m |
| 3 | 10 | 1.2 |
| 4 | 10 | n |
��1���ϱ���m����ֵ ��
��2����Ʒ��̼��Ƶ�����������
��3��Ҫ��ȡ4.4g������̼���躬̼�����������Ϊ80%��ʯ��ʯ���ٿˣ�
ijͬѧȡ�ؿ�ʯ��Ʒ��������Ʒ�е�̼��Ƶ������������м�⣬�����ǣ�ȡ��ʯ��Ʒ8g����40g������Һ���Ĵμ��룬�����������±�����֪ʯ��ʯ��Ʒ�е����ʲ�����ˮ���������ᷴӦ������㣺
| ��� | ����ϡ�����������g�� | ʣ������������g�� |
| 1 | 10 | 5.5 |
| 2 | 10 | m |
| 3 | 10 | 1.2 |
| 4 | 10 | n |
��1���ϱ���m����ֵ ��
��2����Ʒ��̼��Ƶ�����������
��3��Ҫ��ȡ4.4g������̼���躬̼�����������Ϊ80%��ʯ��ʯ���ٿˣ�
ijͬѧȡ�ؿ�ʯ��Ʒ��������Ʒ�е�̼��Ƶ������������м�⣬�����ǣ�ȡ��ʯ��Ʒ8g����40g������Һ���Ĵμ��룬�����������±�����֪ʯ��ʯ��Ʒ�е����ʲ�����ˮ���������ᷴӦ������㣺
| ��� | ����ϡ�����������g�� | ʣ������������g�� |
| 1 | 10 | 5.5 |
| 2 | 10 | m |
| 3 | 10 | 1.2 |
| 4 | 10 | n |
��1���ϱ���m����ֵ ��
��2����Ʒ��̼��Ƶ�����������
��3��Ҫ��ȡ4.4g������̼���躬̼�����������Ϊ80%��ʯ��ʯ���ٿˣ�