��Ŀ����
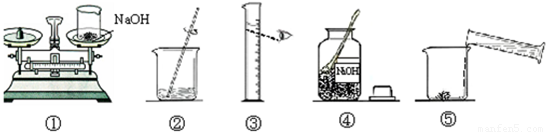
С��Ҫ��100 g��������Ϊ10%������������Һ��������Ҷ����ǩ������ͼ������������������Һ��ʵ�����ʾ��ͼ��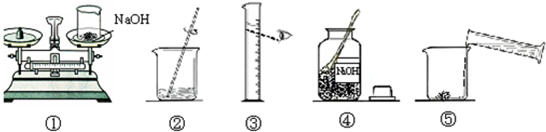
��1��д��ͼ�����ֲ������������ƣ�
��2����ȡ����Ҫ��ˮӦѡ��
��3��ָ��ͼ�е�һ����������
��4��������ͼʾ����ű�ʾ������Һ�IJ���˳��
��5����õ�����������Һ����û�м�ʱװ���Լ�ƿ����ɱ��ʣ��˷�Ӧ�Ļ�ѧ����ʽΪ

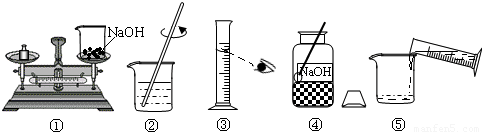
��1��д��ͼ�����ֲ������������ƣ�
�ձ�
�ձ�
����Ͳ���������ɣ�
��Ͳ���������ɣ�
����2����ȡ����Ҫ��ˮӦѡ��
100mL
100mL
��ѡ�10 mL������50 mL����100 mL������Ͳ����3��ָ��ͼ�е�һ����������
�ٳ�������ʱҩƷ������ߵ��������ˮʱ���Ӷ�����
�ٳ�������ʱҩƷ������ߵ��������ˮʱ���Ӷ�����
���������������Ƶ���Һ��������������ƫС
ƫС
���ƫ��ƫС�����䡱������4��������ͼʾ����ű�ʾ������Һ�IJ���˳��
�ܢ٢ۢݢ�
�ܢ٢ۢݢ�
����5����õ�����������Һ����û�м�ʱװ���Լ�ƿ����ɱ��ʣ��˷�Ӧ�Ļ�ѧ����ʽΪ
2NaOH+CO2=Na2CO3+H2O
2NaOH+CO2=Na2CO3+H2O
����������1�����ݳ��������ش�
��2������100g��������Ϊ10%������������Һ��Ҫȷ����ѡ����Ͳ�����ȱ���֪����Ҫȡˮ��������ٸ��ݡ�һ���������ԭ��ѡȡ��Ͳ��
��2��ʹ����ƽʱ��Ҫ��ѭ���������ԭ��ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ��Ӷ�����ɶ�����ȷ����ϸ�۲�ͼ������ȷ������Ĵ𰸣�3��������Һ��һ�㲽��Ϊ���㡢�������ܽ⡢װƿ��ǩ��ŵȣ����Ա���Ĵ𰸺���������ѡ��
��2������100g��������Ϊ10%������������Һ��Ҫȷ����ѡ����Ͳ�����ȱ���֪����Ҫȡˮ��������ٸ��ݡ�һ���������ԭ��ѡȡ��Ͳ��
��2��ʹ����ƽʱ��Ҫ��ѭ���������ԭ��ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ��Ӷ�����ɶ�����ȷ����ϸ�۲�ͼ������ȷ������Ĵ𰸣�3��������Һ��һ�㲽��Ϊ���㡢�������ܽ⡢װƿ��ǩ��ŵȣ����Ա���Ĵ𰸺���������ѡ��
����⣺��1��ͼ�����ڲ������������ձ�������������Ͳ��
�ʴ�Ϊ���ձ�����Ͳ���������ɣ���
��2������100g��������Ϊ10%������������Һ�������������Ƶ�����Ϊ100g��10%=10g����Ҫˮ������Ϊ100g-10g=90g����ˮһ�����������ķ���������ˮ�����Ϊ90mL��ѡ����Ͳʱ��Ӧ��ѡȡ��һ����ȡ������ȡҺ���������ӽ���
�ʴ�Ϊ��100mL��
��3���ù�������������Һ��һ�㲽��Ϊ���㡢�������ܽ⡢װƿ��ǩ��ţ�
�ʴ�Ϊ���ܢ٢ۢݢڣ�
��4��ʹ����ƽʱ��Ҫ��ѭ���������ԭ��ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ�ʹ��ȡ��ˮ����ƫ��ʹ������Һ���ʵ���������ƫС��
�ʴ�Ϊ������ʱҩƷ������ߵ�������ˮʱ���Ӷ�������ƫС��
��5�������������������̼������ѧ��Ӧ����̼���ƺ�ˮ�����ʣ�
�ʴ�Ϊ��2NaOH+CO2=Na2CO3+H2O��
�ʴ�Ϊ���ձ�����Ͳ���������ɣ���
��2������100g��������Ϊ10%������������Һ�������������Ƶ�����Ϊ100g��10%=10g����Ҫˮ������Ϊ100g-10g=90g����ˮһ�����������ķ���������ˮ�����Ϊ90mL��ѡ����Ͳʱ��Ӧ��ѡȡ��һ����ȡ������ȡҺ���������ӽ���
�ʴ�Ϊ��100mL��
��3���ù�������������Һ��һ�㲽��Ϊ���㡢�������ܽ⡢װƿ��ǩ��ţ�
�ʴ�Ϊ���ܢ٢ۢݢڣ�
��4��ʹ����ƽʱ��Ҫ��ѭ���������ԭ��ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ�ʹ��ȡ��ˮ����ƫ��ʹ������Һ���ʵ���������ƫС��
�ʴ�Ϊ������ʱҩƷ������ߵ�������ˮʱ���Ӷ�������ƫС��
��5�������������������̼������ѧ��Ӧ����̼���ƺ�ˮ�����ʣ�
�ʴ�Ϊ��2NaOH+CO2=Na2CO3+H2O��
������������Ҫ����������һ������������������Һ��������⣬������ƽ����Ͳ����ȷʹ�÷���������������ȷ���Ļ����ؼ���������̼��������������Һ��Ӧ�������ʣ�

��ϰ��ϵ�д�
 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
�����Ŀ