��Ŀ����
����ѧ���Ļ�ѧ֪ʶ���ش��������⣺
��1����¶��ˮ���͡�
��2������ʱ�����������������������������д�������������Ϊͭ������仰��ָ���������Ӧұ������ͭ���÷�Ӧ�Ļ�ѧ����ʽΪ
��3��ij������2.3g��ȫȼ�գ���Ҫ4.8������ͬʱ���ɶ�����̼4.4g��ˮ����û�������һ������
��1����¶��ˮ���͡�
���������غ㶨�ɿ�֪��Ӧǰ��Ԫ�ص�����䣬ˮ������ɵ�Ԫ�����ͬ����ˮ�����ܱ����
���������غ㶨�ɿ�֪��Ӧǰ��Ԫ�ص�����䣬ˮ������ɵ�Ԫ�����ͬ����ˮ�����ܱ����
��������»�����˵���ƽ�ѧ���ʺ�������
������
����2������ʱ�����������������������������д�������������Ϊͭ������仰��ָ���������Ӧұ������ͭ���÷�Ӧ�Ļ�ѧ����ʽΪ
CuSO4+Fe�TCu+FeSO4
CuSO4+Fe�TCu+FeSO4
���÷�Ӧ�Ļ��������û���Ӧ
�û���Ӧ
��3��ij������2.3g��ȫȼ�գ���Ҫ4.8������ͬʱ���ɶ�����̼4.4g��ˮ����û�������һ������
C��H��O
C��H��O
Ԫ�أ���������1�����������غ㶨�ɽ�¶ˮ��ȼ�͵IJ���ѧ�ԣ����ݽ��ڸ���ʱ�������仯�ж�����ǿ����
��2��ʪ����ͭ��ԭ������������ͭ��Ӧ���ӷ�Ӧǰ�����ʵ������ص��ж��䷴Ӧ�Ļ������ͣ�
��3�������л���ȼ�����ɶ�����̼��ˮ��֪�л�����һ������C��H����Ԫ�أ��ҿɼ����ˮ������������4.4g������̼��2.7gˮ����C��H����Ԫ�ص���������C��H����Ԫ�ص���������2.3g�����л��ﲻ����OԪ�أ���C��H����Ԫ�ص�����С��2.3g�����л����к���OԪ�أ�
��2��ʪ����ͭ��ԭ������������ͭ��Ӧ���ӷ�Ӧǰ�����ʵ������ص��ж��䷴Ӧ�Ļ������ͣ�
��3�������л���ȼ�����ɶ�����̼��ˮ��֪�л�����һ������C��H����Ԫ�أ��ҿɼ����ˮ������������4.4g������̼��2.7gˮ����C��H����Ԫ�ص���������C��H����Ԫ�ص���������2.3g�����л��ﲻ����OԪ�أ���C��H����Ԫ�ص�����С��2.3g�����л����к���OԪ�أ�
����⣺��1�����û�ѧ��Ӧǰ��Ԫ�ص��������Բ���ˮ��ȼ�ͣ��ƽ��ڸ���ʱ��������Ӧ˵���仯ѧ���ʽ��ȶ���
��2��ʪ����ͭ��ԭ���ǽ���������Һ��Ӧ�е���������ͭ�ķ�Ӧ��������ͭ��������������Ӧ���ص��ǵ����뻯�������ɵ����뻯��������û���Ӧ��Ҫ��
��3���⣺���������غ㶨�ɿ�֪2.3gij�л�����4.8g�������г��ȼ�գ�����4.4g������̼��ͬʱ������ˮ�������ǣ�2.3g+4.8g-4.4g=2.7g��������һ������C��H����Ԫ�أ���̼��Ԫ�ص������ֱ��ǣ�
CԪ�ص�����Ϊ4.4g��
��100%=1.2g��
HԪ�ص�����Ϊ2.7g��
��100%=0.3g��
1.2g+0.3g=1.5g��2.3g��
���л�����һ������OԪ�أ�
�ʴ�Ϊ��
��1�����������غ㶨�ɿ�֪��Ӧǰ��Ԫ�ص�����䣬ˮ������ɵ�Ԫ�����ͬ����ˮ�����ܱ���ͣ������ã�
��2��CuSO4+Fe�TCu+FeSO4���û���Ӧ��
��3��C��H��O
��2��ʪ����ͭ��ԭ���ǽ���������Һ��Ӧ�е���������ͭ�ķ�Ӧ��������ͭ��������������Ӧ���ص��ǵ����뻯�������ɵ����뻯��������û���Ӧ��Ҫ��
��3���⣺���������غ㶨�ɿ�֪2.3gij�л�����4.8g�������г��ȼ�գ�����4.4g������̼��ͬʱ������ˮ�������ǣ�2.3g+4.8g-4.4g=2.7g��������һ������C��H����Ԫ�أ���̼��Ԫ�ص������ֱ��ǣ�
CԪ�ص�����Ϊ4.4g��
| 12 |
| 44 |
HԪ�ص�����Ϊ2.7g��
| 2 |
| 18 |
1.2g+0.3g=1.5g��2.3g��
���л�����һ������OԪ�أ�
�ʴ�Ϊ��
��1�����������غ㶨�ɿ�֪��Ӧǰ��Ԫ�ص�����䣬ˮ������ɵ�Ԫ�����ͬ����ˮ�����ܱ���ͣ������ã�
��2��CuSO4+Fe�TCu+FeSO4���û���Ӧ��
��3��C��H��O
������������Ҫ�Ƕ������غ㶨�ɵĿ��飬ֻ����������������غ㶨�ɵ��������˳�����⣮

��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
��9�֣�ˮ������֮Դ��Ҳ������������Դ������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
(1)��Լˮ��Դ����ֹˮ��Ⱦ��ÿ������Ӧ�������κ������������������ˮ����Ⱦ���У�����ţ� ��
| A����ҵ��ˮֱ���ŷ� | B����ҵ�����������ŷ� |
| C����ֹʹ�ú���ϴ�·� | D������ʹ�û��ʡ�ũҩ |
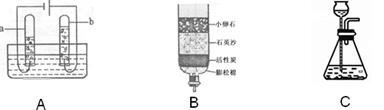
(2)ͼA��ˮͨ��ֽ��ʾ��ͼ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ����ʵ������У��Թ�b�в����������� ��ʵ���ҿ�ѡ��ͼC��ȡ�����壬��װ���з�Ӧ�Ļ�ѧ����ʽΪ�� ��
(3)Ϊ��ȥˮ�еIJ��������ʣ�ijͬѧ������ͼB��ʾ�ļ���ˮ�������л���̿����Ҫ������ ��
(4)ijЩ�ط�������ˮ�к���������Ca(HCO3)2����ˮʱ��Ca(HCO3)2�����ֽⷴӦ�����������Ե�CaCO3��CO2��H2O����д��Ca(HCO3)2���ȷֽ�Ļ�ѧ����ʽ ��