��Ŀ����
��2012?������һģ����ѧ�������غ㶨����һ���dz���Ҫ�Ķ������ɣ�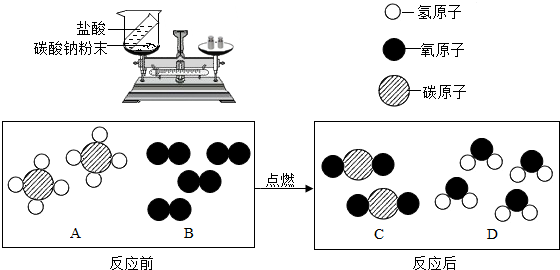
��1����ͼװ���У�����С�ձ����������ʵ�����m1��Ȼ��С�ձ��е�̼������������ȫ��ϣ���Ӧ����һ��ʱ����ٳ���С�ձ�����ƿ�����ʵ�������Ϊm2�����=����������������m1
��2����Ȼ������Ҫ�ɷ��Ǽ��飨CH4������ֱ����������ȼ�ϣ�ȼ�շ�Ӧǰ���������仯����ʾ��ͼ��ͼ��ʾ��
��1��B�������
���������������ڻ��������
��3��A��B�ɷ������·�Ӧ��3A+2B=A3B2��ijѧ������3�θ�ʵ�飨ÿ�ξ���ַ�Ӧ������ӦǰA��B�������Ͷ���l0g���й�ʵ�����ݼ��±���
X��Y�ı�ֵ����Ϊ
��4��Ϊ�˲ⶨijƷ��ʳ�ô�����̼���Ƶ�����������ijУ��ѧ�о���ѧϰС���̽���������£�
��������⡿��Ʒ��̼���Ƶ����������Ƕ��٣�
��֪ʶ����ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ���Ӧ�����в�����ˮ���Ȼ���Ļӷ���
����Ʒ�����
��1������һ����һ������Ʒ�м����������ʯ��ˮ�����ݷ�Ӧ����̼��Ƶ������������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
��2������������һ������Ʒ�м���������ϡ���ᣬ���ݷ�Ӧ���ɶ�����̼�������������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
������ʵ�顿����ͬѧ����ȡ24.00g��Ʒ����ˮ�����Һ������Һ�м�������ij���ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����20.00g������ͬѧ����ȡ24.00g��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���8.80g������̼��
��������⡿������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ�������������д��������̣���������ȷ��0.1% ��

��1����ͼװ���У�����С�ձ����������ʵ�����m1��Ȼ��С�ձ��е�̼������������ȫ��ϣ���Ӧ����һ��ʱ����ٳ���С�ձ�����ƿ�����ʵ�������Ϊm2�����=����������������m1
��
��
m2����ԭ��Ϊ���ɵĶ�����̼�����ݳ��������ձ���������������
���ɵĶ�����̼�����ݳ��������ձ���������������
����2����Ȼ������Ҫ�ɷ��Ǽ��飨CH4������ֱ����������ȼ�ϣ�ȼ�շ�Ӧǰ���������仯����ʾ��ͼ��ͼ��ʾ��
��1��B�������
2
2
��ԭ�ӣ����������������ڻ��������
ACD
ACD
����ͼ����ĸ������3��A��B�ɷ������·�Ӧ��3A+2B=A3B2��ijѧ������3�θ�ʵ�飨ÿ�ξ���ַ�Ӧ������ӦǰA��B�������Ͷ���l0g���й�ʵ�����ݼ��±���
| ��� | ��ӦǰA������ | ��ӦǰB������ | ��Ӧ��A3B2������ |
| �� | 8g | 2g | 6g |
| �� | 4g | 6g | 6g |
| �� | xg | yg | 9g |
7��3
7��3
��3��2
3��2
����4��Ϊ�˲ⶨijƷ��ʳ�ô�����̼���Ƶ�����������ijУ��ѧ�о���ѧϰС���̽���������£�
��������⡿��Ʒ��̼���Ƶ����������Ƕ��٣�
��֪ʶ����ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ���Ӧ�����в�����ˮ���Ȼ���Ļӷ���
����Ʒ�����
��1������һ����һ������Ʒ�м����������ʯ��ˮ�����ݷ�Ӧ����̼��Ƶ������������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
��2������������һ������Ʒ�м���������ϡ���ᣬ���ݷ�Ӧ���ɶ�����̼�������������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
������ʵ�顿����ͬѧ����ȡ24.00g��Ʒ����ˮ�����Һ������Һ�м�������ij���ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����20.00g������ͬѧ����ȡ24.00g��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���8.80g������̼��
��������⡿������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ�������������д��������̣���������ȷ��0.1% ��
88.3%
88.3%
����������1���۲췴Ӧ��װ�ã����ݷ�Ӧ���ڳ��ڵ�װ���н��У�̼���������ᷴӦ���ɵĶ�����̼�������뵽�����У�������Ӧǰ�������ı仯��
��2���۲췴Ӧ����ʾ��ͼ���������ʵĹ��ɣ������ж����ʵ����
��3�����ݵڢٴη�Ӧ�͵ڢڴη�Ӧ���бȽϣ�����������������ͬ��ÿһ�η�Ӧʱ��һ�����ʹ����������������μӷ�Ӧ��A��B�������ȣ��ٸ��ݷ�ӦǰA��B�������Ͷ���l0g������x��y��ֵ��
��4�������������ѡ��ͬѧ��ʵ�����ݽ��м��㣮��̼��Ƴ���������������̼�������������Ʒ�Ӧ�Ļ�ѧ����ʽ���̼���Ƶ����������������Ʒ��̼���Ƶ�����������
��2���۲췴Ӧ����ʾ��ͼ���������ʵĹ��ɣ������ж����ʵ����
��3�����ݵڢٴη�Ӧ�͵ڢڴη�Ӧ���бȽϣ�����������������ͬ��ÿһ�η�Ӧʱ��һ�����ʹ����������������μӷ�Ӧ��A��B�������ȣ��ٸ��ݷ�ӦǰA��B�������Ͷ���l0g������x��y��ֵ��
��4�������������ѡ��ͬѧ��ʵ�����ݽ��м��㣮��̼��Ƴ���������������̼�������������Ʒ�Ӧ�Ļ�ѧ����ʽ���̼���Ƶ����������������Ʒ��̼���Ƶ�����������
����⣺��1�����ڷ�Ӧ���ڳ��ڵ�װ���н��У�̼���������ᷴӦ���ɵĶ�����̼�����ݳ��������ձ��������������ᣮ���ԣ���ӦǰС�ձ����������ʵ��������ڷ�Ӧ��С�ձ����������ʵ���������m1��m2��
��2���������ʵ���ʾ��ͼ��֪��1��B�����к���2��ԭ�ӣ�
�������ʵ���ʾ��ͼ��֪������������A��C��D�����ɲ�ͬԪ����ɵĴ�������ڻ����
��3���������ͼʾ���ݿ�֪����ͬѧ����ҩƷ��ַ�Ӧʱ������������Ϊ6.0g��A��������ȫ��Ӧ����ôB��ȫ��Ӧ��A��ʣ�࣬�ʲ��뷴Ӧ��A������Ϊ��6g-2g=4.0g�����뷴Ӧ��A��������B��������A3B2��=4��2��6��������ͬѧ����ҩƷ��ַ�Ӧʱ��A��ȫ��Ӧ��B��ʣ�ࣻ
A��������B��������A3B2��=4��2��6�����Ԣ�ѧ�����B��ַ�Ӧ����B������Ϊ��
yg��9g=2��6 ��֮�ã�y=3g��
��Ӧ��x��������=10g-3g=7g ��x��y=7��3
���A��ȫ��Ӧ����A������Ϊ��
xg��9g=4��6 ��֮�ã�x=6g��
��Ӧ��y�������ǣ�y=10g-6g=4g����x��y=6��4=3��2��
��4��������20.00g̼�������̼���Ƶ�����Ϊx������
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100
x 20.00g
=
x=21.2g
̼���Ƶ����������ǣ�
��100%=88.3%��
�ʴ�Ϊ����1���������ɵĶ�����̼�����ݳ��������ձ����������������2��2��ACD����3��7��3 ��3��2 ��4��88.3%��
��2���������ʵ���ʾ��ͼ��֪��1��B�����к���2��ԭ�ӣ�
�������ʵ���ʾ��ͼ��֪������������A��C��D�����ɲ�ͬԪ����ɵĴ�������ڻ����
��3���������ͼʾ���ݿ�֪����ͬѧ����ҩƷ��ַ�Ӧʱ������������Ϊ6.0g��A��������ȫ��Ӧ����ôB��ȫ��Ӧ��A��ʣ�࣬�ʲ��뷴Ӧ��A������Ϊ��6g-2g=4.0g�����뷴Ӧ��A��������B��������A3B2��=4��2��6��������ͬѧ����ҩƷ��ַ�Ӧʱ��A��ȫ��Ӧ��B��ʣ�ࣻ
A��������B��������A3B2��=4��2��6�����Ԣ�ѧ�����B��ַ�Ӧ����B������Ϊ��
yg��9g=2��6 ��֮�ã�y=3g��
��Ӧ��x��������=10g-3g=7g ��x��y=7��3
���A��ȫ��Ӧ����A������Ϊ��
xg��9g=4��6 ��֮�ã�x=6g��
��Ӧ��y�������ǣ�y=10g-6g=4g����x��y=6��4=3��2��
��4��������20.00g̼�������̼���Ƶ�����Ϊx������
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100
x 20.00g
| 106 |
| 100 |
| X |
| 20.00g |
̼���Ƶ����������ǣ�
| 21.2g |
| 24.00g |
�ʴ�Ϊ����1���������ɵĶ�����̼�����ݳ��������ձ����������������2��2��ACD����3��7��3 ��3��2 ��4��88.3%��
������������Ҫ����ѧ����������֮��������ȣ�����δ֪�����ʵ�����������������Ĺؼ��Ǹ�����֪�����������֮��������ȣ�Ҫȫ�濼�ǿ��ܴ��ڵ������

��ϰ��ϵ�д�
�����Ŀ



 ��2012?������һģ���������ƣ�CaO2���㷺Ӧ����ˮ����ֳ����ˮ�����������������Ĺ�������
��2012?������һģ���������ƣ�CaO2���㷺Ӧ����ˮ����ֳ����ˮ�����������������Ĺ�������