��Ŀ����
11�� ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺��1����ѧ���š�3H2O��������������ˮ����
��2��ͼ1��ˮͨ��ֽ��ʾ��ͼ����ʵ������У��Թ�a�в���������������������д���÷�Ӧ�Ļ�ѧ����ʽ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2����
��3����ȥˮ�������Թ������ʳ��õIJ����ǹ��ˣ�Ϊ�˾���ˮ��ijͬѧ������ͼ2��ʾ�ļ���ˮ�������������˻���̿������ �ԣ�
��4������ˮ��Ӳˮ������ˮ�����õ������Ƿ���ˮ������˵��������ʹӲˮ������һ�ֳ��÷�����У�
��5��ijЩ�ط�������ˮ�к���������Ca��HCO3��2�ȿ������Σ���ˮʱ��Ca��HCO3��2�����ֽⷴӦ�����������Ե�̼��ơ�ˮ�Ͷ�����̼������д��Ca��HCO3��2���ȷֽ�Ļ�ѧ����Ca��HCO3��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O��
���� ��1�����ݻ�ѧʽ�����������
��2�����ݵ��ˮ������ͽ��۷����ش�
��3�����ݹ��˵�ԭ��������̿�������Է����ش�
��4������Ӳˮ����ˮ�ļ���Ӳˮ���������������ش�
��5��������Ӧ�ķ�Ӧ�������д����Ӧ�Ļ�ѧ����ʽ
��� �⣺��1����3H2O���������ǣ�����ˮ���ӣ�
��2����ˮͨ��ֽ��ʾ��ͼ��֪���Թ�a�в����������ǵ�Դ�ĸ�������������϶࣬�������������÷�Ӧ�Ļ�ѧ����ʽ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2����
��3����ȥˮ�������Թ������ʳ��õIJ����ǹ��ˣ�Ϊ�˾���ˮ��ijͬѧ������ͼ2��ʾ�ļ���ˮ�������������˻���̿������ �ԣ�
��4������ˮ��Ӳˮ������ˮ�����õ������Ƿ���ˮ��������ˮ��������ĭ�ٵ���Ӳˮ��������ˮ��������ĭ�������ˮ��������ʹӲˮ������һ�ֳ��÷�����У�
��5��Ca��HCO3��2���ȷֽ�����̼��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Ca��HCO3��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O��
�ʴ�Ϊ����1������ˮ���ӣ���2����������3�����ˣ���������4������ˮ����У���5����Ca��HCO3��2���ȷֽ�����̼��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Ca��HCO3��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O��
���� �����Ϊȫ��ؿ������й�ˮ��֪ʶ���ѶȲ��������е�֪ʶ������ɣ�

 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�| A�� | ͨ��ˮ�ĵ��ʵ��֤����ˮ�����⡢������Ԫ����ɵ� | |
| B�� | ���ʯ��ʯī��̼�ĵ��ʣ���C60����̼�Ļ����� | |
| C�� | �����ж�����̼���ĵ�;����Ҫ����ɫֲ��Ĺ������ | |
| D�� | �����г�����еķ�������ˮ��Ӳ�� |
| A�� | 5.3g | B�� | ?5.1g | C�� | 5.0g | D�� | ?4.9g |
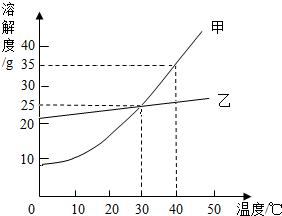 ��ͼ�Ǽס������������ʵ��ܽ�����¶ȱ仯������ͼ���ݴ����ܻش��������⣺
��ͼ�Ǽס������������ʵ��ܽ�����¶ȱ仯������ͼ���ݴ����ܻش��������⣺