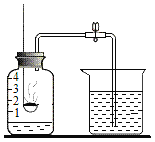
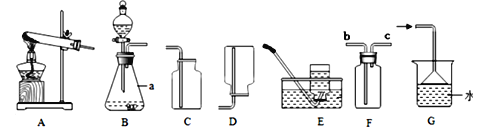
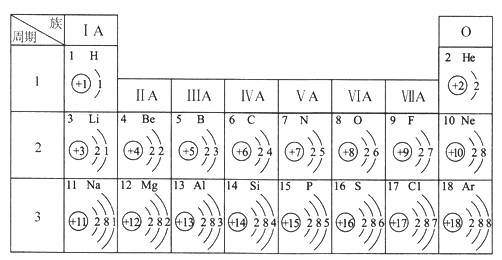
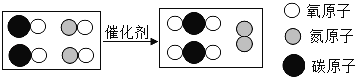
��Ŀ����
����Ŀ��(1)������ѧ��ѧ֪ʶ�����������⣺
���������γ���������Ĥ�Ļ�ѧ����ʽ__________�� ��![]() ___��
___��
(2) ��������(NaN3)���㷺Ӧ����������ȫ���ҡ��������Ƶ��Ʊ�������������Һ̬����Ӧ��NaNH2���ٽ�NaNH2��N2O��Ӧ������NaN3���÷�Ӧ�Ļ�ѧ����ʽΪ2NaNH2 + N2O=NaN3 + NaOH + NH3����ش��������⣺
��N2O��������____________��Һ̬������________(�����������������������)��
��NaNH2�е�Ԫ�صĻ��ϼ�Ϊ____________������H��N��NaԪ�ص�������Ϊ_____��
��NaN3�е�������Ϊ______��������ײ����30������NaN3Ѹ�ٷֽ�ΪNa��N2���䷴Ӧ�Ļ�ѧ����ʽΪ________________________________��
���𰸡� 4Al +3O2![]() 2Al2O3 ��+2�۵���Ԫ�� һ����������һ������������ ������ -3��
2Al2O3 ��+2�۵���Ԫ�� һ����������һ������������ ������ -3��![]() 2��14��23 Na+ 2NaN3
2��14��23 Na+ 2NaN3![]() 2Na+3N2��
2Na+3N2��
��������(1)�������γ���������Ĥ�Ļ�ѧ����ʽΪ��4Al +3O2=2Al2O3���� ![]() ����������+2�۵���Ԫ����(2)��N2O�ɱ�ʾһ����������һ��������������Һ̬������һ��������ɣ����ڴ��������Ԫ����+1�ۣ���Ԫ����+1�ۣ��赪Ԫ�صĻ��ϼ���x�������ڻ��������������ϼ۴�����Ϊ�㣬�ɵã���+1��+x+��+1����2=0����x=-3�ۣ�Na��N��HԪ�ص�������Ϊ23��14��2����������ײ����30������NaN3Ѹ�ٷֽ�ΪNa��N2�����Է�Ӧ����ʽΪ2NaN3
����������+2�۵���Ԫ����(2)��N2O�ɱ�ʾһ����������һ��������������Һ̬������һ��������ɣ����ڴ��������Ԫ����+1�ۣ���Ԫ����+1�ۣ��赪Ԫ�صĻ��ϼ���x�������ڻ��������������ϼ۴�����Ϊ�㣬�ɵã���+1��+x+��+1����2=0����x=-3�ۣ�Na��N��HԪ�ص�������Ϊ23��14��2����������ײ����30������NaN3Ѹ�ٷֽ�ΪNa��N2�����Է�Ӧ����ʽΪ2NaN3![]() 2Na+3N2����
2Na+3N2����