��Ŀ����
��160����������Ϊ10%������ͭ��Һ�У���������һ����������������������Һ����������������Һ�����������ɳ����������Ĺ�ϵ���±���| �� �� | ��һ�� | �ڶ��� | ������ | ���IJ� |
| ��������������Һ���������ˣ� | 50 | 75 | 120 | 160 |
| �����������������ˣ� | 4.9 | 7.35 | 9.8 | 9.8 |
��2������ͭ��Һ�����ʵ�������______
��3���μӵ���______��ʱ����������ͭ�Ѿ���ȫ��Ӧ��
��4������������Һ������������
���𰸡���������1����������ͭ���������Ƶķ�Ӧ��д����Ӧ�ķ���ʽ��
��2���ɵ������Ĵ��������ݿ�֪���������������Ƶ����������ɳ����������������ӣ�˵������Һ�е�����ͭ��ȫ����Ӧ���ɳ������������������ͭ��Һ�����ʵ�������
��3�����ݵ������Ĵ������֪���������������Ƶ����������ɳ������������ٱ仯��˵���˵μӵ�������ʱ����������ͭ�Ѿ���ȫ��Ӧ��
��4���ɵ�һ�������������ݿ�֪��50g����������Һ��ȫ��Ӧʱ����������ͭ����������Ϊ4.9g�����ݷ�Ӧ�ķ���ʽ��������ͭ�������������������������Һ������������
����⣺��1������ͭ���������Ƶķ�Ӧ�����������ƺ�������ͭ��������Ӧ�ķ���ʽ�ǣ�CuSO4+2NaOH=Na2SO4+Cu��OH��2��
��2��������ͭ��Һ�����ʵ�����ΪX
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 98
X 9.8g
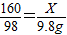 ��ã�X=16g
��ã�X=16g
��3���ɵ������Ĵ������֪���������������Ƶ����������ɳ������������ٱ仯��˵���˵μӵ�������ʱ����������ͭ�Ѿ���ȫ��Ӧ��
��4����50g����������Һ�����ʵ�����Ϊy
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
80 98
y 4.9g
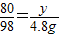 ��ã�y=4g
��ã�y=4g
����������Һ����������Ϊ��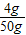 ×100%=8%
×100%=8%
�ʴ�Ϊ����1��CuSO4+2NaOH=Na2SO4+Cu��OH��2���� ��2��16�ˣ� ��3��������4��8%��
������������Ҫ�����йػ�ѧ����ʽ�ļ�����й��������������ļ��㣬�ѶȽϴ������ݶԱ��ҳ�������Ϣ���ǽ���Ĺؼ���
��2���ɵ������Ĵ��������ݿ�֪���������������Ƶ����������ɳ����������������ӣ�˵������Һ�е�����ͭ��ȫ����Ӧ���ɳ������������������ͭ��Һ�����ʵ�������
��3�����ݵ������Ĵ������֪���������������Ƶ����������ɳ������������ٱ仯��˵���˵μӵ�������ʱ����������ͭ�Ѿ���ȫ��Ӧ��
��4���ɵ�һ�������������ݿ�֪��50g����������Һ��ȫ��Ӧʱ����������ͭ����������Ϊ4.9g�����ݷ�Ӧ�ķ���ʽ��������ͭ�������������������������Һ������������
����⣺��1������ͭ���������Ƶķ�Ӧ�����������ƺ�������ͭ��������Ӧ�ķ���ʽ�ǣ�CuSO4+2NaOH=Na2SO4+Cu��OH��2��
��2��������ͭ��Һ�����ʵ�����ΪX
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 98
X 9.8g
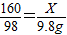 ��ã�X=16g
��ã�X=16g��3���ɵ������Ĵ������֪���������������Ƶ����������ɳ������������ٱ仯��˵���˵μӵ�������ʱ����������ͭ�Ѿ���ȫ��Ӧ��
��4����50g����������Һ�����ʵ�����Ϊy
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
80 98
y 4.9g
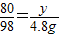 ��ã�y=4g
��ã�y=4g����������Һ����������Ϊ��
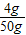 ×100%=8%
×100%=8%�ʴ�Ϊ����1��CuSO4+2NaOH=Na2SO4+Cu��OH��2���� ��2��16�ˣ� ��3��������4��8%��
������������Ҫ�����йػ�ѧ����ʽ�ļ�����й��������������ļ��㣬�ѶȽϴ������ݶԱ��ҳ�������Ϣ���ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
��160����������Ϊ10%������ͭ��Һ�У���������һ����������������������Һ����������������Һ�����������ɳ����������Ĺ�ϵ���±���
| �������������� | ��һ�� | �ڶ��� | ������ | ���IJ� |
| ��������������Һ���������ˣ� | 50 | 75 | 120 | 160 |
| �����������������ˣ� | 4.9 | 7.35 | 9.8 | 9.8 |
��2������ͭ��Һ�����ʵ�������________
��3���μӵ���________��ʱ����������ͭ�Ѿ���ȫ��Ӧ��
��4������������Һ������������