题目内容
(10分)太阳能光伏发电最关键的材料是高纯硅。硅的原子结构简图为: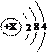
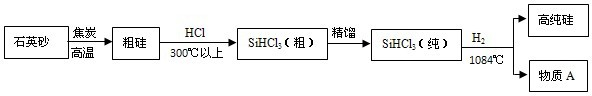
 纯硅是通过以下过程制取的:(一)制粗硅:
纯硅是通过以下过程制取的:(一)制粗硅: (二)制纯硅:② Si(粗)+ 2Cl2
 SiCl4 ③ SiCl4 + 2H2
SiCl4 ③ SiCl4 + 2H2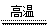 Si(纯)+ 4HCl
Si(纯)+ 4HCl纯净的无色透明的硅的氧化物(SiO2)晶体就是通常所说的水晶,这也许你不熟悉,但说起沙子,你一定不会陌生,沙子的主要成份即是SiO2,SiO2的化学性质与CO2有些类似。请根据以上信息,回答以下问题:
(1)X的值为 。
(2)推断SiO2的物理性质(三点即可) 、 、 。
(3)请写出SiO2与NaOH溶液反应的化学方程式: 。
(4)在反应①②③中,②属于 反应类型,属于置换反应的是(填序号) 。
(5)化学上将只要有元素的化合价发生了变化的化学反应就叫做氧化还原反应,氧化还原反应中含有化合价升高了的元素的反应物叫还原剂,反应①中的还原剂是(写化学式) 。
(10分)
(1) 14
(2) 无色透明、不溶于水、硬度大、熔点高、固体(任意三点,但答“黄色”不给分)
(3) SiO2 + 2NaOH = Na2SiO3 + H2O
(4) 化合反应 ①③ (2分,每个1分) (5) C 解析:
略
(1) 14
(2) 无色透明、不溶于水、硬度大、熔点高、固体(任意三点,但答“黄色”不给分)
(3) SiO2 + 2NaOH = Na2SiO3 + H2O
(4) 化合反应 ①③ (2分,每个1分) (5) C 解析:
略

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目