题目内容
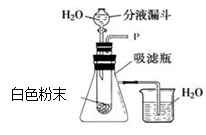
某气体可能由以下气体中的一种或几种组成:①水蒸气②CO③H2④CO2。某同学设计了如图示的装置进行实验,A、B、C、D瓶可根据需要盛装物质。请根据要求回答问题:

(1)如果此气体是由①②③三种气体组成,若用干燥的还原性气体和CuO反应,且能观察到A瓶中固体物质有明显的颜色变化,则A瓶装的固体物质可能是 (填化学式)。(2)如果此气体是由①②③④四种气体组成,则B瓶装的物质可能是
(填物质名称),C瓶装的物质可能是 (填物质名称)。
(3)当经过A、B、C装置后的干燥气体与CuO在加热的条件下充分反应,若D瓶中没有发生明显变化(D瓶中装有与A装中相同的固体物质),E瓶中有沉淀产生。则原混合气体中一定不含 (填气体编号)。
(4)如果有①②③④四种气体经过A、B、C装置,且B瓶中盛装的物质是碱溶液,请写出在B瓶中发生的化学反应方程式: ,该设计的实验装置有不完善的地方是 。

(1)如果此气体是由①②③三种气体组成,若用干燥的还原性气体和CuO反应,且能观察到A瓶中固体物质有明显的颜色变化,则A瓶装的固体物质可能是 (填化学式)。(2)如果此气体是由①②③④四种气体组成,则B瓶装的物质可能是
(填物质名称),C瓶装的物质可能是 (填物质名称)。
(3)当经过A、B、C装置后的干燥气体与CuO在加热的条件下充分反应,若D瓶中没有发生明显变化(D瓶中装有与A装中相同的固体物质),E瓶中有沉淀产生。则原混合气体中一定不含 (填气体编号)。
(4)如果有①②③④四种气体经过A、B、C装置,且B瓶中盛装的物质是碱溶液,请写出在B瓶中发生的化学反应方程式: ,该设计的实验装置有不完善的地方是 。
(1)CuSO4 (2)澄清石灰水 浓硫酸 (3)③
(4)CO2+Ca(OH)2=CaCO3↓+H2O 尾气没有处理
(4)CO2+Ca(OH)2=CaCO3↓+H2O 尾气没有处理
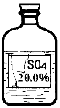
(1)具有还原性的气体有CO和H2,无水硫酸铜白色固体遇水则变成蓝色进行解答
(2)根据实验目的是还原氧化铜,要先把气体中的水和二氧化碳除掉进行解答
(3)根据由实验装置可知E是除掉反应生成的二氧化碳,D是除掉反应生成的水,进行解答
(4)根据B瓶中盛装的物质是碱溶液,是除掉二氧化碳进行解答
(2)根据实验目的是还原氧化铜,要先把气体中的水和二氧化碳除掉进行解答
(3)根据由实验装置可知E是除掉反应生成的二氧化碳,D是除掉反应生成的水,进行解答
(4)根据B瓶中盛装的物质是碱溶液,是除掉二氧化碳进行解答

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目