��Ŀ����
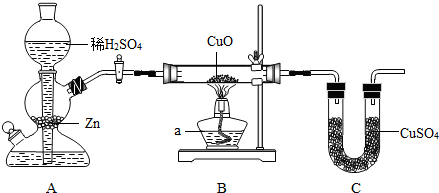
��2011?�������ģ����ѧ��ȤС���ͬѧ����ͼ��װ�ò���ˮ���⡢��Ԫ�ص������ȣ��䷽���Ƿֱ�ⶨͨ����ǰ��Bװ�ò����ܵ��������U�ܵ������Ȼ�����ȷ��ˮ���⡢��Ԫ�ص������ȣ�
��ش��������⣺
��д��װ���У�����a������
��װ��B�IJ������ڿɹ۲쵽��������
������װ������������п��ܴ��ڵİ�ȫ������
��������ˮ��m��H����m��O��=1��8����ʵ����m��H����m��O����1��8����������ǿ�����ˮ�ֵ�Ӱ�죬����������һ����Ĺؼ�ԭ����

��ش��������⣺
��д��װ���У�����a������
�ƾ���
�ƾ���
��װ��A���������շ�����
���շ�����
����װ���ڷ�Ӧ�Ļ�ѧ����ʽΪZn+H2SO4=ZnSO4+H2��
Zn+H2SO4=ZnSO4+H2��
����װ��B�IJ������ڿɹ۲쵽��������
��ɫ���ɫ
��ɫ���ɫ
��������Ӧ�Ļ�ѧ����ʽΪH2+CuO
Cu+H2O
| ||
H2+CuO
Cu+H2O
���÷�Ӧ��������������������
| ||
����ͭ
����ͭ
��װ��C��U����CuSO4�������������Ƿ���ˮ����
�����Ƿ���ˮ����
��������װ������������п��ܴ��ڵİ�ȫ������
����ը
����ը
��������ķ�������ͨ���������ټ���
��ͨ���������ټ���
����������ˮ��m��H����m��O��=1��8����ʵ����m��H����m��O����1��8����������ǿ�����ˮ�ֵ�Ӱ�죬����������һ����Ĺؼ�ԭ����
�����к���ˮ����
�����к���ˮ����
���Ľ�����������������
����������
��������ʵ��������п��ϡ�����ڳ���������ȡ�����ģ����շ�����������ʱ���Ʒ�Ӧ�Ľ��У��������п�ȼ�Ժͻ�ԭ�ԣ����ʹ������ʱ��Ҫע���鴿������������ͭ�ڼ��ȵ������·�Ӧ����ͭ��ˮ����������ԭ��������ͭ������������ˮ����ͭһ���Ǽ����Ƿ���ˮ���ɵģ�ע���ʵ�鷽���ͽ��۵����ۣ�
����⣺��1���ƾ����dz��õļ������������շ�����������ʱ���Ʒ�Ӧ�Ľ��У�п�����ᷴӦ��������п���������ʴ�Ϊ���ƾ��ƣ����շ�������Zn+H2SO4=ZnSO4+H2��
��2��װ��B�IJ������ڿɹ۲쵽�������ǣ���ɫ���ɫ������������ͭ�ڼ��ȵ������·�Ӧ����ͭ��ˮ�������ǻ�ԭ��������ͭ������������ˮ����ͭһ���Ǽ����Ƿ���ˮ���ɣ��ʴ�Ϊ����ɫ���ɫ��H2+CuO
Cu+H2O������ͭ�������Ƿ���ˮ����
��3���������п�ȼ�ԣ����������������ը�����Ӧ��ͨ���������ž��������ټ��ȣ��ʴ�Ϊ������ը����ͨ���������ټ���
��4�������к���ˮ���������Cװ�����ӵ�������ƫ��Cװ���Dz�����Ԫ�صģ������Ԫ��������ƫ�����ʵ����m��H����m��O����1��8���Ľ������ǣ�����������ʴ�Ϊ�������к���ˮ����������������
��2��װ��B�IJ������ڿɹ۲쵽�������ǣ���ɫ���ɫ������������ͭ�ڼ��ȵ������·�Ӧ����ͭ��ˮ�������ǻ�ԭ��������ͭ������������ˮ����ͭһ���Ǽ����Ƿ���ˮ���ɣ��ʴ�Ϊ����ɫ���ɫ��H2+CuO
| ||
��3���������п�ȼ�ԣ����������������ը�����Ӧ��ͨ���������ž��������ټ��ȣ��ʴ�Ϊ������ը����ͨ���������ټ���
��4�������к���ˮ���������Cװ�����ӵ�������ƫ��Cװ���Dz�����Ԫ�صģ������Ԫ��������ƫ�����ʵ����m��H����m��O����1��8���Ľ������ǣ�����������ʴ�Ϊ�������к���ˮ����������������
����������������ʵ��̽���⣬�����������ȡ���������Ļ�ԭ�ԣ�����������ļ���Ͷ�ʵ������ۣ��ۺ��ԱȽ�ǿ�����������Ŀ�ṩ����Ϣ����Ͽα���ѧ����֪ʶ���������Ҫע�⻯ѧ����ʽ����д����ƽ����������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ