题目内容
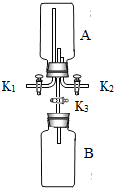
下图为实验室制取气体的装置图,回答下列问题: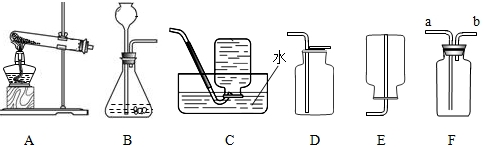
(1)用高锰酸钾制取氧气时选用的发生装置为
(2)利用B图气体发生装置能够制备的常见气体有
写出制备上述气体的化学方程式:
(3)氨气密度比空气小,且易溶于水,水溶液呈碱性.实验室通常用固体氯化铵与熟石灰混合加热制得,制取氨气时应选择的装置组合是
分析:根据图示可分析得:A为固体加热制取气体的装置.B用于固体与液体不加热制取气体的反应.C为排水法收集气体的装置,所收集气体要不溶于水不与水反应.D为向上排空气法收集气体的装置,所收集的气体密度比空气大且不与空气中的成分反应.E为向下排空气法收集气体的装置,所收集的气体密度比空气小且不与空气中的成分反应.F可作为排空气法收集气体的装置(密度大于空气的气体收集时从a进气,密度小于空气的气体收集时从b进气),亦可作洗气装置.
解答:解:(1)高锰酸钾制氧气为固体加热制取气体的反应,而氧气不溶于水,密度略大于空气,故收集时可采用排水法或向上排空气法,若选用F应从a端进气,故答案为:A C a
(2)B图用于固体与液体不加热制取气体的反应,初中阶段接触到的固体与液体不加热制取的气体有:双氧水制氧气,活泼金属与稀酸反应制氢气,石灰石与稀盐酸制二氧化碳.
故答案为:O2、CO2或H2、O2或H2、CO2 2H2O
2H2O+O2↑CaCO3+2HCl=CaCl2+H2O+CO2↑ Zn+2HCl=ZnCl2+H2↑
(3)氨气制取时采用固体氯化铵与熟石灰加热,故选择的制取装置为固体加热装置,而氨气密度小于空气,易溶于水,故所采用的收集方法为向下排空气法,故答案为:AE
(2)B图用于固体与液体不加热制取气体的反应,初中阶段接触到的固体与液体不加热制取的气体有:双氧水制氧气,活泼金属与稀酸反应制氢气,石灰石与稀盐酸制二氧化碳.
故答案为:O2、CO2或H2、O2或H2、CO2 2H2O
| ||
(3)氨气制取时采用固体氯化铵与熟石灰加热,故选择的制取装置为固体加热装置,而氨气密度小于空气,易溶于水,故所采用的收集方法为向下排空气法,故答案为:AE
点评:本题考查了制取气体时发生装置和收集装置的选择以及由装置考虑所能制取的气体和反应的原理,对学生来讲要熟悉常见气体的制取原理和性质,再根据原理和性质选用发生装置和收集装置,甚至于中间的除杂装置.

练习册系列答案
相关题目
 、
、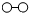 、
、 分别表示N2、H2、NH3.在催化剂表面(
分别表示N2、H2、NH3.在催化剂表面( 下图中表示催化剂表面)N2和H2反应合成氨的反应过程可用五张图表示如下:
下图中表示催化剂表面)N2和H2反应合成氨的反应过程可用五张图表示如下:
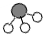
 微粒的基本性质”的实验探究,实验过程如下:
微粒的基本性质”的实验探究,实验过程如下:
 、
、 、
、 分别表示N2、H2、NH3分子.在催化剂表面
分别表示N2、H2、NH3分子.在催化剂表面 (下图中表示催化剂表面)N2和H2反应合成氨的反应过程可用五张图表示如下:
(下图中表示催化剂表面)N2和H2反应合成氨的反应过程可用五张图表示如下: