��Ŀ����
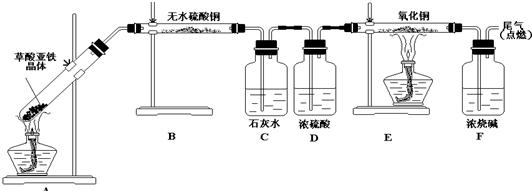
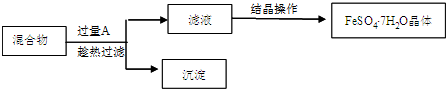
 �������ĿҪ��ش��������⣮
�������ĿҪ��ش��������⣮��1����ͼΪ������ƽ��ʹ��������ƽ����Ʒ����ȷ����˳��Ϊ������ţ�
B
B
���ٵ����� �ڷų���ֽ �۳���������� �ܵ���� �ݼ������������ ������С������
A���٢ڢۢܢݢ�B���ܢڢۢݢޢ�C���ܢݢޢ٢ڢ�
��2����С�ձ��м�������ͭ��Һ��������������������Ϊm1��������Ӧ��ʵ������Ϊ
�����ϸ��ź�ɫ���ʣ���Һ����ɫ��Ϊdz��ɫ
�����ϸ��ź�ɫ���ʣ���Һ����ɫ��Ϊdz��ɫ
_����Ӧ������һ��ʱ����ٳ���С�ձ����ձ������ʵ�������Ϊ m2�����=����������������m1=
=
m2����3������ͼװ���У�����С�ձ����������ʵ�����m1��Ȼ��С�ձ��е�̼������������ȫ��ϣ�������Ӧ�Ļ�ѧ����ʽΪ
Na2CO3+2HCl=2NaCl+H2O+CO2��
Na2CO3+2HCl=2NaCl+H2O+CO2��
����Ӧ������һ��ʱ����ٳ���С�ձ�����ƿ�����ʵ�������Ϊ m2�����=����������������m1��
��
m2����ԭ��Ϊ���ɵĶ�����̼��ɢ�������У����������غ㶨�ɣ���Ӧǰ�ձ��з�Ӧ������������ڷ�Ӧ���ձ���ʣ�����������
���ɵĶ�����̼��ɢ�������У����������غ㶨�ɣ���Ӧǰ�ձ��з�Ӧ������������ڷ�Ӧ���ձ���ʣ�����������
����4����4A+5B=4C+6D�ķ�Ӧ�У�C��D��Է�������֮��Ϊ15��9������1.7g A��B��ȫ��Ӧ����3gC����B��C��������Ϊ
4��3
4��3
����������1����������ƽ��������ʱ����ȷ����˳����з������
��2��������ɫ������ͭ��Һ�����û���Ӧ������������������ˮ�У�ͬʱ���ɺ�ɫ����ͭ����Ӧ��û������μӣ����Է��ڳ��ڵ��ձ��н���ʵ�飬��Ӧǰ���ձ�����������Ҳ���䣮
��3��̼���������ᷴӦ�������������������̼�����Է���С�ձ��ڽ��з�Ӧʱ��������̼������ɢ�������У���ʹ��Ӧǰ���ձ������ʵ���������ȣ�
��4�����ݻ�ѧ����ʽ����֪������C��D��Է�������֮��Ϊ15��9������1.7g A��B��ȫ��Ӧ����3gC�����г�����ʽ���������D��������Ȼ����������غ㶨�ɼ����B�����������������B��C�������ȣ�
��2��������ɫ������ͭ��Һ�����û���Ӧ������������������ˮ�У�ͬʱ���ɺ�ɫ����ͭ����Ӧ��û������μӣ����Է��ڳ��ڵ��ձ��н���ʵ�飬��Ӧǰ���ձ�����������Ҳ���䣮
��3��̼���������ᷴӦ�������������������̼�����Է���С�ձ��ڽ��з�Ӧʱ��������̼������ɢ�������У���ʹ��Ӧǰ���ձ������ʵ���������ȣ�
��4�����ݻ�ѧ����ʽ����֪������C��D��Է�������֮��Ϊ15��9������1.7g A��B��ȫ��Ӧ����3gC�����г�����ʽ���������D��������Ȼ����������غ㶨�ɼ����B�����������������B��C�������ȣ�
����⣺��1������ƽ��������ʱ����ȷ���������ǣ��ȵ���㣬Ȼ�������������и���һ����ͬ��ֽ���ѳ�����ŵ����̣�Ȼ���������з����룬�������˳�����ȷ�������ģ��ٷ�����С�ģ�����������ƽ������ȷ����˳��Ϊ�ܢڢۢݢޢ٣�
��2����������ͭ��Һ�����û���Ӧ����������������ͭ������ͭ��Һ����ɫ������������ҺΪdz��ɫ�����ɺ�ɫ��ͭ�����������棻��Ӧ��û������μӣ������ձ������ʵ�������ʼ�ղ��䣮
��3��̼���������������Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+H2O+CO2�������������غ㶨�ɣ���Ӧǰ�����ʵ����������ڷ�Ӧ������ʵ������������������ɵĶ�����̼����ų��������ձ������ʵ��������淴Ӧ�ķ������ϼ�С��
��4���⣺��֪C��D����Է���������Ϊ15��9��������D������Ϊx����
4A+5B=4C+6D
15��4 9��6
3g x
=
x=2.7g
���������غ㶨�ɿɵòμӷ�Ӧ��B������=3g+2.7g-1.7g=4g��
��B��C��������Ϊ4g��3g=4��3��
�ʴ�Ϊ����1��B��
��2�������ϸ��ź�ɫ���ʣ���Һ����ɫ��Ϊdz��ɫ��=��
��3��Na2CO3+2HCl=2NaCl+H2O+CO2�����������ɵĶ�����̼��ɢ�������У����������غ㶨�ɣ���Ӧǰ�ձ��з�Ӧ������������ڷ�Ӧ���ձ���ʣ�������������
��3��4��3��
��2����������ͭ��Һ�����û���Ӧ����������������ͭ������ͭ��Һ����ɫ������������ҺΪdz��ɫ�����ɺ�ɫ��ͭ�����������棻��Ӧ��û������μӣ������ձ������ʵ�������ʼ�ղ��䣮
��3��̼���������������Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+H2O+CO2�������������غ㶨�ɣ���Ӧǰ�����ʵ����������ڷ�Ӧ������ʵ������������������ɵĶ�����̼����ų��������ձ������ʵ��������淴Ӧ�ķ������ϼ�С��
��4���⣺��֪C��D����Է���������Ϊ15��9��������D������Ϊx����
4A+5B=4C+6D
15��4 9��6
3g x
| 15��4 |
| 9��6 |
| 3g |
| x |
���������غ㶨�ɿɵòμӷ�Ӧ��B������=3g+2.7g-1.7g=4g��
��B��C��������Ϊ4g��3g=4��3��
�ʴ�Ϊ����1��B��
��2�������ϸ��ź�ɫ���ʣ���Һ����ɫ��Ϊdz��ɫ��=��
��3��Na2CO3+2HCl=2NaCl+H2O+CO2�����������ɵĶ�����̼��ɢ�������У����������غ㶨�ɣ���Ӧǰ�ձ��з�Ӧ������������ڷ�Ӧ���ձ���ʣ�������������
��3��4��3��
�����������ѶȲ�������������غ㶨�ɵ�Ӧ�ã�����Ĺؼ��Ƿ����������ݣ�������������غ㶨�ɣ���Ҫע�����κ�����Χ��������ϵ�У����۷������ֱ仯����̣���������ʼ�ձ��ֲ��䣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
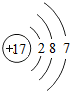
�±��ǵ������ڲ���Ԫ�ص���Ϣ���ϣ��������ĿҪ��ش��������⣮
��1���ڱ�����Ӧ�ĺ����ϣ���д��Ӧ�����ݣ�
| Ԫ������ | Ԫ�ط��� | ԭ�ӽṹʾ��ͼ | ��ߡ���ͻ��ϼ� | ��� |
| �� | Na |  | +1 | ���� |
| þ | Mg |  | +2 | ���� |
________ | Al |  | +3 | ���� |
| �� | Si |  | +4��-4 | ________ |
| �� | P |  | +5��-3 | �ǽ��� |
| �� | S | 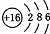 | +6��________ | �ǽ��� |
| �� | ________ |  | +7��-1 | �ǽ��� |








 �������ĿҪ��ش��������⣮
�������ĿҪ��ش��������⣮