��Ŀ����
��ʵ��̨�ϰڷ�������������ҩƷ��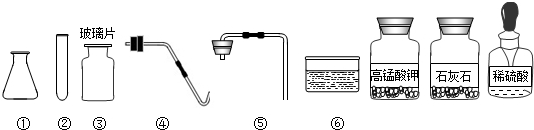
��1�������ṩ��������ҩƷ������ȡ��������
��2����������������ҩƷ��ȡ������Ӧѡ�������Ϊ
��3�������������еIJ�����
��4�������ṩ��ҩƷ�������Ƶó���ѧϰ������һ�����壬��Ӧ�Ļ�ѧ����ʽΪ
��5���������⣨2������ѡ������Ƶ���ȡ�����װ�ã��㷢�������ڵ�ȱ����
��������1����ҩƷȷ��������ʵ�飻
��2�����Ҫ����ʵ��ȷ��ʵ����Ʒ�������������ģ�
��3�����������̼�ó���ʯ��ˮ��
��4����Ӧ�˵�һ���⣬���������ȡ������������ȣ�
��5����װ����û�з�Һ©�����Ͳ��ܿ��Ʒ�Ӧ���ʣ�
��2�����Ҫ����ʵ��ȷ��ʵ����Ʒ�������������ģ�
��3�����������̼�ó���ʯ��ˮ��
��4����Ӧ�˵�һ���⣬���������ȡ������������ȣ�
��5����װ����û�з�Һ©�����Ͳ��ܿ��Ʒ�Ӧ���ʣ�
����⣺��1����ҩƷȷ��������ʵ�飻��Ϊû�м���װ�ã�����ֻ����ȡ������̼��
��2�����Ҫ����ʵ��ȷ��ʵ����Ʒ�������������ģ�
��3�����������̼�������Ļ�ѧ�����������Һ��Ӧ������ͨ������ʯ��ˮ�����Ƿ����ǣ�
��4����ҩƷ�ɿ������������ȡ����������������̨�̶����м��ȣ�
��5��װ����û�з�Һ©�������ܿ��Ʒ�Ӧ���ʣ�Ҳ������ʱ����Һ�壮
�ʴ�Ϊ����1��CO2��CaCO3+2HCl=CaCl2+CO2��+H2O��
��2���٢ۢݣ���ڢۢݣ���
��3��ͨ�����ʯ��ˮ���Ƿ����ǣ�
��4��2KMnO4
K2MnO4+MnO2+O2�����ƾ��ƣ�����̨��
��5��װ����û�з�Һ©�������ܿ��Ʒ�Ӧ���ʣ�Ҳ������ʱ����Һ�壮
��2�����Ҫ����ʵ��ȷ��ʵ����Ʒ�������������ģ�
��3�����������̼�������Ļ�ѧ�����������Һ��Ӧ������ͨ������ʯ��ˮ�����Ƿ����ǣ�
��4����ҩƷ�ɿ������������ȡ����������������̨�̶����м��ȣ�
��5��װ����û�з�Һ©�������ܿ��Ʒ�Ӧ���ʣ�Ҳ������ʱ����Һ�壮
�ʴ�Ϊ����1��CO2��CaCO3+2HCl=CaCl2+CO2��+H2O��
��2���٢ۢݣ���ڢۢݣ���
��3��ͨ�����ʯ��ˮ���Ƿ����ǣ�
��4��2KMnO4
| ||
��5��װ����û�з�Һ©�������ܿ��Ʒ�Ӧ���ʣ�Ҳ������ʱ����Һ�壮
������������к�ǿ���ۺ��ԣ��漰��ʵ�����ĵ�ѡȡ��ʵ��װ�õ�����˳��ѧ����ʽ����д�Լ������ռ��ļ���ȣ�����Ĺؼ���ȷ����ʵ����Ƶ�Ŀ�ģ���ȷ�Ľ��ʵ����������ų����������ӭ�ж��⣮

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ


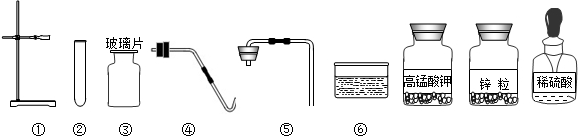
 ��
��