��Ŀ����
��1��������ͼ��ʾ����������գ�
�ٹ���ʱ���õ�����
�ڿ�ֱ�Ӽ��ȵ���
�ۿ������ռ��������������
�ܿ�����������Һ�ͽϴ����Լ��ķ�Ӧ��������
��2����ͼ�ǻ�ѧʵ���г��õļ���װ�ã���ش���������
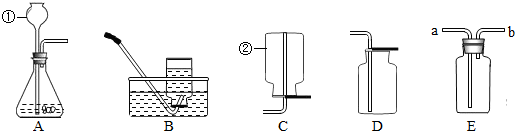
����������A��Dװ�õ���Ͽ�����ȡ��һ��������
��ijͬѧ���ռ�һ�����������壬��ΪEװ�ÿ��Դ���Bװ�ã��������ͬѧӦ���ʹ��Eװ�ã�
��������1���ɳ�����������;���з�����ɣ�
��2�������ݷ���װ�ú��ռ�װ�õ�ѡ���������
�������ռ������ԭ��˼�����
��2�������ݷ���װ�ú��ռ�װ�õ�ѡ���������
�������ռ������ԭ��˼�����
����⣺��1�����ݻ�ѧʵ�鳣��������;��֪��
�ٹ���ʱ���õ�����©����
�ڿ�ֱ�Ӽ��ȵ��������Թܡ�������
�ۿ������ռ�������������Ǽ���ƿ��
�ܿ�����������Һ�ͽϴ����Լ��ķ�Ӧ���������ձ���
��2����Aװ�����ڹ�Һ����װ�ã�Dװ���������ſ��������ռ������ܶȱȿ���������壬���Կ�ѡ�������̼������������˫��ˮ�����������ȡ��������
�ڸ�װ������ˮ���ռ��������һ��װ�ã������뵹��ʹ�÷�����ͬ��Eװ�ó���ˮ��������b�˽��룬������������࣬�ڲ�ѹǿ��������ʹҺ���a��ȥ������Eװ�ó���ˮ�ţ�������a�˽��룩��
�ʴ�Ϊ��
��1����C ��E ��D ��A��
��2����CO2��CaCO3+2HCl=CaCl2+CO2��+H2O������O2��2H2O2
2H2O+O2������
�ڼ���ƿ��װ��ˮ�������b�ڽ�����Eװ�ó���ˮ�ţ�������a�˽��룩��
�ٹ���ʱ���õ�����©����
�ڿ�ֱ�Ӽ��ȵ��������Թܡ�������
�ۿ������ռ�������������Ǽ���ƿ��
�ܿ�����������Һ�ͽϴ����Լ��ķ�Ӧ���������ձ���
��2����Aװ�����ڹ�Һ����װ�ã�Dװ���������ſ��������ռ������ܶȱȿ���������壬���Կ�ѡ�������̼������������˫��ˮ�����������ȡ��������
�ڸ�װ������ˮ���ռ��������һ��װ�ã������뵹��ʹ�÷�����ͬ��Eװ�ó���ˮ��������b�˽��룬������������࣬�ڲ�ѹǿ��������ʹҺ���a��ȥ������Eװ�ó���ˮ�ţ�������a�˽��룩��
�ʴ�Ϊ��
��1����C ��E ��D ��A��
��2����CO2��CaCO3+2HCl=CaCl2+CO2��+H2O������O2��2H2O2
| ||
�ڼ���ƿ��װ��ˮ�������b�ڽ�����Eװ�ó���ˮ�ţ�������a�˽��룩��
����������Ƚϼ���Ҫ���鳣��ʵ����������;������װ�ú��ռ�װ�õ�ѡȡ��ͬѧ����ƽʱҪ�����ĸ���������;������ѡ��װ�õı���ͬʱѧ����������ռ�װ�ã�

��ϰ��ϵ�д�
�����Ŀ
 ��������
�������� ��1���ر�Aװ���е�ֹˮ�кӳ���©������ƿ��ע��һ������ˮ����ֹ����ͼ��ʾ����Aװ���Ƿ�©����
��1���ر�Aװ���е�ֹˮ�кӳ���©������ƿ��ע��һ������ˮ����ֹ����ͼ��ʾ����Aװ���Ƿ�©����  ���©����������©��������ȷ������
���©����������©��������ȷ������