��Ŀ����
(6��) ˮ���˼�һ����������������ģ�Ϊ���������ᾭ�õĿɳ�����չ������Ӧ���˽��й�ˮ��һЩ֪ʶ������ش�
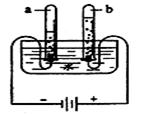
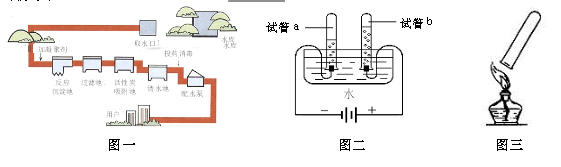
(1)��ͼ�ǵ��ˮʵ��װ�á���ʵ������У��Թ�a������������ ��д��ˮ��ͨ�������·�Ӧ�Ļ�ѧ����ʽ ��
(2)��Ȼˮ�к����������ʣ����������������������˺�����ȷ������������о����̶���ߵķ����� ��
(3)Ӳˮ����������������ܶ��鷳�������п��� ������Ӳˮ����ˮ��
(4)�������Լ��7l����ˮ���ǣ����ɹ��������õĵ�ˮ����ȴ����1��������ˮ��Դ��ÿ����������κ�����������Ϊ���ڽ�Լ��ˮ���� (�����)

(1)��ͼ�ǵ��ˮʵ��װ�á���ʵ������У��Թ�a������������ ��д��ˮ��ͨ�������·�Ӧ�Ļ�ѧ����ʽ ��
(2)��Ȼˮ�к����������ʣ����������������������˺�����ȷ������������о����̶���ߵķ����� ��
(3)Ӳˮ����������������ܶ��鷳�������п��� ������Ӳˮ����ˮ��
(4)�������Լ��7l����ˮ���ǣ����ɹ��������õĵ�ˮ����ȴ����1��������ˮ��Դ��ÿ����������κ�����������Ϊ���ڽ�Լ��ˮ���� (�����)
| A��ϴ�������ʱ��������ͷ | B������Ϸ�ˮˢ�� |
| C��������ˮ����Ϊ���ϳ�ˮ���� | D����ϴ��ˮ����� |
��1��H2���������� 2H2O 2H2��+ O2�� ������������ƽ���������֣�
2H2��+ O2�� ������������ƽ���������֣�
��2������ ��3������ˮ����ϴ�Ӽ��� ��4��D
 2H2��+ O2�� ������������ƽ���������֣�
2H2��+ O2�� ������������ƽ���������֣���2������ ��3������ˮ����ϴ�Ӽ��� ��4��D
�����������1�����ݵ��ˮʵ��ġ��������⣬�����һ�����������������������������������������������������2��1�������ʾ��ͼ��֪��a�Թ�����������ϴ�������������ôb�Թ��е�����Ϊ�������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ2H2O
 2H2��+ O2����
2H2��+ O2������2���������õ���������ˮ�����ڴ�����ʾ����̶���ߡ�
��3�������У�����Ӳˮ����ˮ�����÷���ˮ�������ˮ�������ĭ�������ˮ�����������ĭ�ٵ���Ӳˮ��
��4�����ݽ�Լ��ˮ�Ĵ�ʩ������
A��ϴ�������ʱ��������ͷ����ʹˮ�װ��˷ѣ�����
B������Ϸ�ˮˢ�����Ƕ�ˮ���˷ѣ�����
C��������ˮ����Ϊ���ϳ�ˮ���£��Ƕ�ˮ���˷ѣ�����
D����ϴ��ˮ�����������һˮ���ã���ȷ����ѡD
�����������ѶȲ����漰��֪ʶ��϶࣬���ڶԻ���֪ʶ�Ŀ��飬Ҫ��ѧ����ƽʱ��ѧϰ��Ҫ��ǿ���֪ʶ�Ĵ�����

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ

 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�