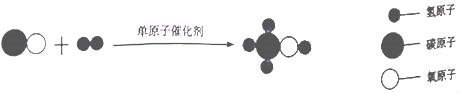
��Ŀ����
����Ŀ����ͼ��ijƷ�Ƶı���ƿ����ش��������⣺

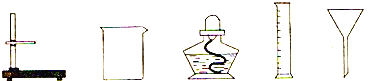
(1)�ñ���ƿ�IJ����Dz���֣����ʲ������___________(�����������������������)��
(2)����������൱�ڹ���ʵ���е�______(��������)��
(3)����(��ѧʽ��CH3COOH)��ϡ���ᶼ�������ͨ�ԣ�����Ϊ������ˮ��Һ���ܵ�������������(CH3COO-)��_______����д��������ᷴӦ���ɴ�������((CH3COO)2Fe)��һ����ɫ����ķ�Ӧ����ʽ:________________________��
(4)�ƳɺϽ�������Ʒ�����һ�ַ�����Ϊ�˷�ֹ����Ʒ���⣬���˽����ƳɺϽ𣬻�����__________________________(д��һ�ַ�������)�ﵽ�����Ŀ�ġ�
���𰸡� ����� ��ֽ H+ 2CH3COOH + Fe = (CH3COO)2Fe + H2 �� Ϳ��(���ᡢ�´ɡ���Ƶ�)
�����������⿼���˺Ͻ𣬽�������ķ�Ӧ�ͽ��������ʩ�ȡ�
��1��������к����������������ǻ���
��2������������൱�ڹ���ʵ���е���ֽ���ܽ�������Һ����룻
��3����������������������ӹ��ɵĻ�������Դ�����ˮ��Һ���ܵ����CH3COO-��H+��������ᷴӦ���ɴ�������((CH3COO)2Fe)����������Ӧ����ʽ��2CH3COOH + Fe = (CH3COO)2Fe + H2 ����
��4��Ϊ�˷�ֹ����Ʒ���⣬���˽����ƳɺϽ𣬻�����Ϳ��(���ᡢ�´ɡ���Ƶ�)��