��Ŀ����
�����ջ�ѧʵ���г�����������������;�������ڿ�չ��ѧѧϰ���о�����������ѡ��ĸ�����գ�

��1����ϸ��ƿ�㵹Һ��ʱ��ƿ��Ҫ
��2����ȡ�͵μ�����Һ���Լ���������
��3����
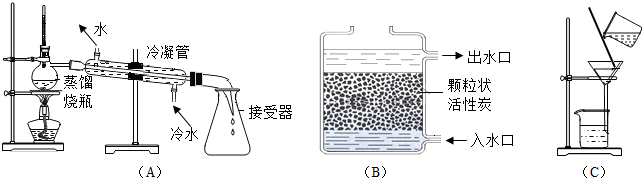
��С���ռ���һƿ���ǵ�ϼ�ֻ��Ǻ�ˮ������ģ������ˮ���ľ�ˮ���̣��������Ƴ�����ˮ�����������ͼ��ʾ����ش��������⣮

��1�������ٵ�������
�����������ٺ�����Һ��C�����л��ǣ���ԭ�������
a��©���ڵ���ֽ������
b��©���¶�δ�����ձ��ڱ�
c��©����Һ�������ֽ�ı�Ե
��2��С��ȡ����Һ��D���Թ��У���������
��3����������Ҫ�dz�ȥһЩ��ζ��ɫ�أ�Ӧѡ�������ĸ�װ��

��1����ϸ��ƿ�㵹Һ��ʱ��ƿ��Ҫ
����
����
�����ϣ���ǩ����
����
���ģ���2����ȡ�͵μ�����Һ���Լ���������
B
B
�������Ҫ��ʱ��ϴ
��ϴ
����3����
H
H
����ȡһ����Һ��ʱ��������Һ��İ�Һ�����ʹ�
��ʹ�
����ˮƽ����С���ռ���һƿ���ǵ�ϼ�ֻ��Ǻ�ˮ������ģ������ˮ���ľ�ˮ���̣��������Ƴ�����ˮ�����������ͼ��ʾ����ش��������⣮

��1�������ٵ�������
����
����
���ò����б����õ��IJ���������©�����ձ���������
������
�������������ٺ�����Һ��C�����л��ǣ���ԭ�������
ac
ac
�����ţ���a��©���ڵ���ֽ������
b��©���¶�δ�����ձ��ڱ�
c��©����Һ�������ֽ�ı�Ե
��2��С��ȡ����Һ��D���Թ��У���������
����ˮ
����ˮ
�����۲쵽��ĭ�����н϶�ĸ���������˵��Һ��D��Ӳˮ����3����������Ҫ�dz�ȥһЩ��ζ��ɫ�أ�Ӧѡ�������ĸ�װ��
B
B
�����ţ����ù�����Ҫ������
����
�仯�����������ѧ������
��������1�����ݻ�ѧʵ��Ļ���������Ҫ��������в�����
��2��������������;���ش�
��3��������Ͳ��ʹ�÷�������;���ش�
��1�����˿��Ѳ�����ˮ�����ʳ�ȥ�����ݹ��˲������������ش𣻸��ݲ����Ľ�����ƶ������еIJ��������ݲ���Ҫ�㣬�жϵ��²���ʧ���ԭ��
��2���÷���ˮ���Լ���ˮ����Ӳ��
��3������̿�������ԣ��û���̿����ˮ�е�ɫ�غ���ζ��ȥ��
��2��������������;���ش�
��3��������Ͳ��ʹ�÷�������;���ش�
��1�����˿��Ѳ�����ˮ�����ʳ�ȥ�����ݹ��˲������������ش𣻸��ݲ����Ľ�����ƶ������еIJ��������ݲ���Ҫ�㣬�жϵ��²���ʧ���ԭ��
��2���÷���ˮ���Լ���ˮ����Ӳ��
��3������̿�������ԣ��û���̿����ˮ�е�ɫ�غ���ζ��ȥ��
����⣺��1����ϸ��ƿ�㵹Һ��ķ�����ƿ��Ҫ���������ϣ���ǩ�������ģ�������š�����
��2����ͷ�ιܿ�����ȡ�͵μ�����Һ���Լ��������Ҫƽ���������ϣ�����մ���˽�ͷ�ιܣ�Ҫ��ʱ��ϴ�����B����ϴ��
��3����Ͳ������ȡһ������Һ�壬��ȡʱ������Һ��İ�Һ�����ʹ�����ˮƽ�����H����ʹ���
�⣺��1��������ˮ��Ϊ�˽�С�������������������������������ʹ�õ���������������Ϊ�˳�ȥ������ˮ�Ĺ������ʣ������ù��˵ķ���������ʱ�IJ���������©�����ձ��Ͳ����������˺��Ի��ǣ����ж��л����δ�����˶�ֱ��������Һ�У�������©���ڵ���ֽ���������©����Һ�������ֽ�ı�Ե������ɻ���ﲻ�����˶�ֱ�����£�������ˡ���������ac��
��2������Ӳˮ�к��н϶�Ŀ����Եĸơ�þ��������μӷ���ˮʱ����ֺܶ�ĸ���������Һ��D����Ӳˮ�����������ɽ�Ӳˮ�����ˮ��������ˮ�к��еĸơ�þ��������ٻ�û�У��ͻ���ָ����٣���ĭ������������ˮ��
��3���ڹ��˺��ˮ����Ȼ���п����Ե���ɫ������ζ�����ʣ�ͨ����������������������������ǿ���ǻ���̿���ñ仯����û�������������ʣ��������������仯�����B��������
��2����ͷ�ιܿ�����ȡ�͵μ�����Һ���Լ��������Ҫƽ���������ϣ�����մ���˽�ͷ�ιܣ�Ҫ��ʱ��ϴ�����B����ϴ��
��3����Ͳ������ȡһ������Һ�壬��ȡʱ������Һ��İ�Һ�����ʹ�����ˮƽ�����H����ʹ���
�⣺��1��������ˮ��Ϊ�˽�С�������������������������������ʹ�õ���������������Ϊ�˳�ȥ������ˮ�Ĺ������ʣ������ù��˵ķ���������ʱ�IJ���������©�����ձ��Ͳ����������˺��Ի��ǣ����ж��л����δ�����˶�ֱ��������Һ�У�������©���ڵ���ֽ���������©����Һ�������ֽ�ı�Ե������ɻ���ﲻ�����˶�ֱ�����£�������ˡ���������ac��
��2������Ӳˮ�к��н϶�Ŀ����Եĸơ�þ��������μӷ���ˮʱ����ֺܶ�ĸ���������Һ��D����Ӳˮ�����������ɽ�Ӳˮ�����ˮ��������ˮ�к��еĸơ�þ��������ٻ�û�У��ͻ���ָ����٣���ĭ������������ˮ��
��3���ڹ��˺��ˮ����Ȼ���п����Ե���ɫ������ζ�����ʣ�ͨ����������������������������ǿ���ǻ���̿���ñ仯����û�������������ʣ��������������仯�����B��������
������������Ҫ�����˳�����������;����ѧʵ��Ļ����������ڽ���ʵ��ʱ��Ӧ�ϸ�Ҫ�淶�������������õ�ʵ��ϰ�ߣ�����Ҫ��ѧ�������û�ѧ��֪ʶ������������н��ͣ�����ȿ������û�ѧ֪ʶ�����ճ�����������������������ֳ���ѧ�����������ߣ�

��ϰ��ϵ�д�
�����Ŀ
��1��ͨ��һ��Ļ�ѧѧϰ�����Ѿ�������ʵ������ȡ������йع��ɣ�����������װ��ͼ�ش����⣺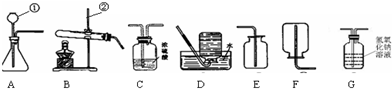
a��д���������������Ƣ�______����______��
b������ͬѧ���ø��������ȡ������Ӧѡ�õķ���װ����______ ������ţ���ͬ����д���÷�Ӧ�Ļ�ѧ����ʽ��______��
c���������������ϣ���֪��Ȳ��C2H2���������ˮ��CaC2���巴Ӧ��ȡ��д���÷�Ӧ�Ļ�ѧ����ʽΪ______������ȡ����Ȳ�����г�����������������CO2��H2S��Ϊ��ȥ����õ������������Ȳ���壬װ������˳��Ϊ��A��______��______��F��
��2�����������ǻ�ѧ�г�����һ��ҩƷ������dz�ư�ɫ������ĩ���ڿ���������ˮ�ֺͶ�����̼��������ˮ����ˮ���ܷ�����Ӧ�����������ƺ������⣬����ʽ��Na2O2+2H2O=2NaOH+H2O2�������������ֽܷ�ų�������
a������ˮ���������ʵ�鷽���ǣ�______�������ķ�Ӧ����ʽ�ǣ�______��
b��ijͬѧΪ��֤���������Ƽ���ˮ���������������ƣ������ɵ���Һ�еμ��˼�����ɫ��̪��Һ���۲쵽����������Һ�ȱ�����Ϊ��ɫ������Դ��������̽����
������⣺ʲô������Һ�ȱ������ɫ��
��������裺
����1����ͬѧ����������ɫ��̪��Һ���ʵ�����Һ��ɫ����
����2����ͬѧ�����������ɵĹ�������ʹ��Һ��ɫ����
ʵ������ۣ�
�ټ�ͬѧ����IJ��룬���������ͬѧ�ķ��ԣ���Ϊ______��
��Ϊ����֤�Լ��IJ��룬��ͬѧ��������·�����ʵ����֤��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ����ϡ����������Һ���Թ��У��μ�1-2�η�̪��Һ������______ �۲����� | ��Һ��ȻΪ��ɫ | ______ |
����3��______��
����3��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ______ | ______ | ______ |