��Ŀ����
��2012?��������ģ�������غ㶨�ɵķ��ֺͷ�չ�Կ�ѧ��չ�����ش����壮
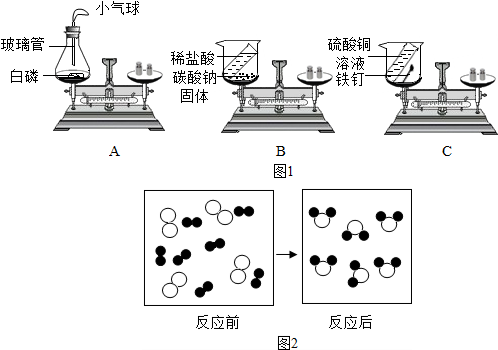
��1������ͼ1��ʾ3��ʵ��ʵ��֤�����غ㶨�ɣ����в��ܴﵽʵ��Ŀ����
��2���ڻ�ѧ��Ӧǰ��һ���仯����
a��ԭ������ b��ԭ����Ŀ c���������� d��������Ŀ e��Ԫ������ f����������
��3����ͼ2��ij��Ӧ����ʾ��ͼ�����С��𡱺͡��ֱ��ʾ��ͬ��ԭ�ӣ���
�ٷ�Ӧ����Ӧ������ ������
������
������۵ĽǶȷ�����ѧ�仯��ʵ����
��4����һ�ܱ���������4������A��B��C��D��������һ�������·�����ѧ��Ӧ������A��B��C����ʾ��ͼ�ͷ�Ӧǰ������ʵ��������±���ʾ��
��A��B��C��D���������������
��x��ֵΪ
����D����Է�������Ϊ46���÷�Ӧ�Ļ�ѧ����ʽ��
��1������ͼ1��ʾ3��ʵ��ʵ��֤�����غ㶨�ɣ����в��ܴﵽʵ��Ŀ����
B
B
������ţ���������������̼���Ʒ�Ӧ�����ɶ�����̼��װ��û���ܷ⣬���Ե�����ƽ����ƽ��
������̼���Ʒ�Ӧ�����ɶ�����̼��װ��û���ܷ⣬���Ե�����ƽ����ƽ��
��
��2���ڻ�ѧ��Ӧǰ��һ���仯����
cf
cf
������ţ���a��ԭ������ b��ԭ����Ŀ c���������� d��������Ŀ e��Ԫ������ f����������
��3����ͼ2��ij��Ӧ����ʾ��ͼ�����С��𡱺͡��ֱ��ʾ��ͬ��ԭ�ӣ���
�ٷ�Ӧ����Ӧ������
2
2
���� ������
������������۵ĽǶȷ�����ѧ�仯��ʵ����
���ӵķֿ�ԭ�ӵ��������
���ӵķֿ�ԭ�ӵ��������
����4����һ�ܱ���������4������A��B��C��D��������һ�������·�����ѧ��Ӧ������A��B��C����ʾ��ͼ�ͷ�Ӧǰ������ʵ��������±���ʾ��
| ���� | A | B | C | D | ��-��ԭ�� ��-��ԭ��  -̼ԭ�� -̼ԭ�� |
| ��ʾ��ͼ |  |
 |
 |
||
| ��Ӧǰ����/g | 100 | 1 | 1 | 46 | |
| ��Ӧ������/g | 4 | 89 | 55 | X |
BC
BC
������ţ�����x��ֵΪ
0
0
������D����Է�������Ϊ46���÷�Ӧ�Ļ�ѧ����ʽ��
C2H5OH+3O2
2CO2+3H2O
| ||
C2H5OH+3O2
2CO2+3H2O
��
| ||
��������1�����������غ㶨�ɵ�Ҫ��ʵ��ʱ��Ӧǰ�����ʵ�������Ӧ����ȣ��ݴ˷�����Ӧǰ�����ʵ������仯���ɣ�
��2���������غ㶨�ɵ��������֪������Ӧǰ��ԭ�ӵ��������Ŀ���䣬�ڻ�ѧ��Ӧǰ��һ��������ǣ�ԭ�����ࡢ��Ŀ�������ݴ˷������ɣ�
��3�����������غ㶨�ɵ��۽����жϣ���Ӧǰ��ԭ�ӵ��������Ŀ��������жϼ��ɣ�
��4���ٸ���������ĸ��������
�ڸ��������غ㶨�ɣ���������������������ȷ����Ӧ�����������������Ӷ��ó�X��ֵ��
�۸���������Ԫ�ص�������ȷ���������ʵ�ԭ�Ӹ����ȣ��Ӷ��õ����ʵĻ�ѧʽ�����ݻ�ѧ����ʽ����д�����д���÷�Ӧ�Ļ�ѧ����ʽ��
��2���������غ㶨�ɵ��������֪������Ӧǰ��ԭ�ӵ��������Ŀ���䣬�ڻ�ѧ��Ӧǰ��һ��������ǣ�ԭ�����ࡢ��Ŀ�������ݴ˷������ɣ�
��3�����������غ㶨�ɵ��۽����жϣ���Ӧǰ��ԭ�ӵ��������Ŀ��������жϼ��ɣ�
��4���ٸ���������ĸ��������
�ڸ��������غ㶨�ɣ���������������������ȷ����Ӧ�����������������Ӷ��ó�X��ֵ��
�۸���������Ԫ�ص�������ȷ���������ʵ�ԭ�Ӹ����ȣ��Ӷ��õ����ʵĻ�ѧʽ�����ݻ�ѧ����ʽ����д�����д���÷�Ӧ�Ļ�ѧ����ʽ��
����⣺��1�����������غ㶨�ɿ�֪����ѧ��Ӧǰ��������ʵ�����֮����ȣ������ƽһֱ����ƽ�⣬˵����Ӧǰ���������䣮���������μӻ������������ɵķ�Ӧһ��Ҫ�ܷ⣬��ֹ�����ȥ��û������ķ�Ӧ�����ܷ⣮�����Ż��ܵͣ�������Ȼ�����������μӷ�Ӧ���������������ļ��٣���������Ҳ���С��������װ���ܱղ����������������ƽƽ�⣻̼���ƺ�ϡ���ᷴӦ�����Ȼ��ƺ�ˮ�Ͷ�����̼��������̼������������У��ᵼ����ƽ��ƽ�⣮����ͭ������������������ͭ��û�������������ɹ���ƽ��Ȼƽ�⣮
��2���������غ㶨�ɵ��������֪���ڻ�ѧ��Ӧǰ��һ��������ǣ�ԭ�����ࣻԭ����Ŀ��ԭ��������һ���仯�������ʵ��������ӵ����࣬�����������п��ܻᷢ���仯��
��3�����������غ㶨�ɣ���Ӧǰ��ԭ�ӵ��������Ŀ���䣬�������� ���Ӹ�ԭ�Ӹ���������ȣ���ͼʾ���Կ�����ѧ�仯��ʵ���ǣ����ӵķֿ�ԭ�ӵ�������ϣ�
���Ӹ�ԭ�Ӹ���������ȣ���ͼʾ���Կ�����ѧ�仯��ʵ���ǣ����ӵķֿ�ԭ�ӵ�������ϣ�
��4������Ӧ���е�������ֻ����Ԫ�أ�����������е�̼Ԫ�غ���Ԫ��һ������M����M��һ������̼������Ԫ�أ�����������Ԫ�ص�����Ϊ88g��
+54g��
=112g��96g����ȷ���������е�һ������Ԫ������D����D��һ��������Ԫ�أ�����D�к�������Ԫ�أ�
����������ĸ����֪��ֻ��BC����������ĸ��
�ڸ��������غ㶨�ɣ��ɱ������ݿ�֪��������̼������������89g-1g=88g����ȷ��������̼�������ˮ����������������55g-1g=54g����ȷ��ˮ�����������
����������������100g-4g=96g��88g+54g����ȷ��������D���Ƿ�Ӧ�D���ٵ�����Ϊ��88g+54g-96g=46g�����X��ֵΪ��46-46=0��
����Ӧ���е�������ֻ����Ԫ�أ�����������е�̼Ԫ�غ���Ԫ��һ������M����M��һ������̼������Ԫ�أ�����������Ԫ�ص�����Ϊ88g��
+54g��
=112g��96g����ȷ���������е�һ������Ԫ������D����D��һ��������Ԫ�أ�D��̼���⡢����Ԫ�ص�������Ϊ88g��
��54g��
����112g-96g��=12��3��8�����D��̼���⡢����Ԫ�ص�ԭ�Ӹ�����Ϊ
��
��
=2��6��1����D�Ļ�ѧʽΪC2H5OH���÷�Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
2CO2+3H2O��
�ʴ�Ϊ����1��B��������̼���Ʒ�Ӧ�����ɶ�����̼��װ��û���ܷ����Ե�����ƽ����ƽ�⣻��2��cf����3����2���ڷ��ӵķֿ�ԭ�ӵ�������ϣ���4��BC��0��C2H5OH+3O2
2CO2+3H2O��
��2���������غ㶨�ɵ��������֪���ڻ�ѧ��Ӧǰ��һ��������ǣ�ԭ�����ࣻԭ����Ŀ��ԭ��������һ���仯�������ʵ��������ӵ����࣬�����������п��ܻᷢ���仯��
��3�����������غ㶨�ɣ���Ӧǰ��ԭ�ӵ��������Ŀ���䣬��������
 ���Ӹ�ԭ�Ӹ���������ȣ���ͼʾ���Կ�����ѧ�仯��ʵ���ǣ����ӵķֿ�ԭ�ӵ�������ϣ�
���Ӹ�ԭ�Ӹ���������ȣ���ͼʾ���Կ�����ѧ�仯��ʵ���ǣ����ӵķֿ�ԭ�ӵ�������ϣ���4������Ӧ���е�������ֻ����Ԫ�أ�����������е�̼Ԫ�غ���Ԫ��һ������M����M��һ������̼������Ԫ�أ�����������Ԫ�ص�����Ϊ88g��
| 16��2 |
| 44 |
| 16 |
| 18 |
����������ĸ����֪��ֻ��BC����������ĸ��
�ڸ��������غ㶨�ɣ��ɱ������ݿ�֪��������̼������������89g-1g=88g����ȷ��������̼�������ˮ����������������55g-1g=54g����ȷ��ˮ�����������
����������������100g-4g=96g��88g+54g����ȷ��������D���Ƿ�Ӧ�D���ٵ�����Ϊ��88g+54g-96g=46g�����X��ֵΪ��46-46=0��
����Ӧ���е�������ֻ����Ԫ�أ�����������е�̼Ԫ�غ���Ԫ��һ������M����M��һ������̼������Ԫ�أ�����������Ԫ�ص�����Ϊ88g��
| 16��2 |
| 44 |
| 16 |
| 18 |
| 12 |
| 44 |
| 2 |
| 18 |
| 12 |
| 12 |
| 3 |
| 1 |
| 8 |
| 16 |
| ||
�ʴ�Ϊ����1��B��������̼���Ʒ�Ӧ�����ɶ�����̼��װ��û���ܷ����Ե�����ƽ����ƽ�⣻��2��cf����3����2���ڷ��ӵķֿ�ԭ�ӵ�������ϣ���4��BC��0��C2H5OH+3O2
| ||
�������������й������غ㶨�ɵĿ��飬����Ĺؼ�������ö��ɵĺ�ۼ��ۺ��壬��һ�������Ϊȫ����й������غ㶨�ɵĺ��⣮

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
�����Ŀ