��Ŀ����
��2013?��������ģ�����������װ�ã��ش�����
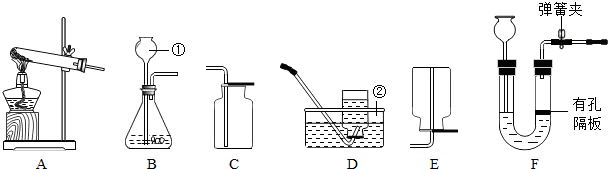
��1��д����Ţ٢ڵ��������ƣ���
��2��д���ø��������ȡO2��Ӧ�Ļ�ѧ����ʽ
��3��ʵ�����ƶ�����̼����ʱ��������ķ���װ����B�Ľ�ΪF���ŵ���
��4����ͬѧ����Bװ�ò����Ķ�����̼����ͨ�����ʯ��ˮ������ʯ��ˮû�б���ǣ�ԭ�������
��5��ʵ�����г��ü����Ȼ�泥�NH4Cl�����������ƹ�������ķ�����ȡ������NH3����ͬʱ�������Ȼ��ƺ�ˮ��д��ʵ������ȡ�����Ļ�ѧ����ʽ
��6��С��ͬѧΪ��̽��������ijЩ���ʣ���������ʵ�飺
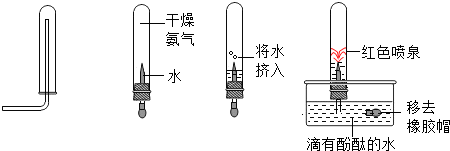
ͼ�д�����������������ʵ�鼰��Ӧ����ͨ��ʵ�飬С��ͬѧ�ɵó�������������

��1��д����Ţ٢ڵ��������ƣ���
����©��
����©��
��ˮ��
ˮ��
����2��д���ø��������ȡO2��Ӧ�Ļ�ѧ����ʽ
2KMnO4
K2MnO4+MnO2+O2��
| ||
2KMnO4
K2MnO4+MnO2+O2��
��
| ||
��3��ʵ�����ƶ�����̼����ʱ��������ķ���װ����B�Ľ�ΪF���ŵ���
������ʱ���Ʒ�Ӧ�ķ�����ֹͣ
������ʱ���Ʒ�Ӧ�ķ�����ֹͣ
����4����ͬѧ����Bװ�ò����Ķ�����̼����ͨ�����ʯ��ˮ������ʯ��ˮû�б���ǣ�ԭ�������
ʹ����Ũ���ᣨ�������ɣ�
ʹ����Ũ���ᣨ�������ɣ�
������ʯ��ˮ�������̼��Ӧ�Ļ�ѧ��Ӧ�ķ���ʽ��Ca��OH��2+CO2=CaCO3��+H20
Ca��OH��2+CO2=CaCO3��+H20
����5��ʵ�����г��ü����Ȼ�泥�NH4Cl�����������ƹ�������ķ�����ȡ������NH3����ͬʱ�������Ȼ��ƺ�ˮ��д��ʵ������ȡ�����Ļ�ѧ����ʽ
2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3��
| ||
2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3��
��
| ||
��6��С��ͬѧΪ��̽��������ijЩ���ʣ���������ʵ�飺

ͼ�д�����������������ʵ�鼰��Ӧ����ͨ��ʵ�飬С��ͬѧ�ɵó�������������
�������ܶȱȿ�����С�������𰸾��ɣ�
�������ܶȱȿ�����С�������𰸾��ɣ�
����дһ������������1����Ϥ�����������˽����ƣ�
��2���ݸ��������ȡ�����ķ�Ӧԭ����д����ʽ��
��3���۲��װ�õ��ص㣬�ᷢ�ֵ��ɼ���������ס�ܵģ��п����������Ź�������ģ�����ѹǿ��ԭ���������ɵ�֪��װ�õ���Խ�ԣ�
��4������ʹ����Ũ���ᣬҲ����ʯ��ˮ���ʵ�ԭ��ʹʯ��ˮδ����ǣ����ݶ�����̼���������Ʒ�Ӧԭ����д����ʽ��
��5������������ṩ�ķ�Ӧ���Ӧ������������д����ѧ��Ӧʽ��
��6��������ͨ��ʵ��ó����ۣ�Ӧ����ʵ��������������ʣ�
��2���ݸ��������ȡ�����ķ�Ӧԭ����д����ʽ��
��3���۲��װ�õ��ص㣬�ᷢ�ֵ��ɼ���������ס�ܵģ��п����������Ź�������ģ�����ѹǿ��ԭ���������ɵ�֪��װ�õ���Խ�ԣ�
��4������ʹ����Ũ���ᣬҲ����ʯ��ˮ���ʵ�ԭ��ʹʯ��ˮδ����ǣ����ݶ�����̼���������Ʒ�Ӧԭ����д����ʽ��
��5������������ṩ�ķ�Ӧ���Ӧ������������д����ѧ��Ӧʽ��
��6��������ͨ��ʵ��ó����ۣ�Ӧ����ʵ��������������ʣ�
����⣺��1����Ţ٢ڵ������ֱ��dz���©����ˮ�ۣ�
��2�����ȸ�����������������������̺�����أ�����ʽ�ǣ�2KMnO4
K2MnO4+MnO2+O2����
��3��װ��F����ȡ����ʱ�������ɼд���Һ�Ӵ��������壬��ס�ܣ�U�ι��ڵ�ѹǿ����Һ��ͺ����ϵĹ���ֿ�����Ӧ�ͻ�ֹͣ�����Ը�װ�þ����濪��ص��ŵ㣻
��4��Ũ�����ӷ����ӷ����Ȼ����������ʯ��ˮ��Ӧ�����Ȼ��ƣ���ʯ��ˮ������ǣ���ʯ��ˮ����Ҳ������ֻ�����������̼��ʯ��ˮ��Ӧ����̼��Ƴ�����ˮ������ʽ�ǣ�Ca��OH��2+CO2=CaCO3��+H20��
��5��������Ϣ��ʵ�����г��ü����Ȼ�泥�NH4Cl�����������ƹ�������ķ�����ȡ������NH3����ͬʱ�������Ȼ��ƺ�ˮ������֪����ȡ�����Ļ�ѧ����ʽ�ǣ�
2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3����
��6���ɵ�һ��ʵ��ͼ��֪�������쵽�������Թܵײ������������ϵ��³����Թܵģ�˵���������ܶȱȿ�����С����ˮ�������İ����У�Ȼ����ȥ��ñ����
���з�̪��ˮ�п�����ɫ��Ȫ��˵���Թ��ڵ�ѹǿ��С���Ұ�����ˮ��Ӧ���������Լ��ԣ��ɴ˿�֪����������ˮ��Ӧ���ɼ������ʣ�
�ʴ�Ϊ����1������©����ˮ�ۣ�
��2��2KMnO4
K2MnO4+MnO2+O2����
��3��������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��4��ʹ����Ũ���ᣨ�������ɣ���Ca��OH��2+CO2=CaCO3��+H20��
��5��2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3����
��6���������ܶȱȿ�����С�������𰸾��ɣ���
��2�����ȸ�����������������������̺�����أ�����ʽ�ǣ�2KMnO4
| ||
��3��װ��F����ȡ����ʱ�������ɼд���Һ�Ӵ��������壬��ס�ܣ�U�ι��ڵ�ѹǿ����Һ��ͺ����ϵĹ���ֿ�����Ӧ�ͻ�ֹͣ�����Ը�װ�þ����濪��ص��ŵ㣻
��4��Ũ�����ӷ����ӷ����Ȼ����������ʯ��ˮ��Ӧ�����Ȼ��ƣ���ʯ��ˮ������ǣ���ʯ��ˮ����Ҳ������ֻ�����������̼��ʯ��ˮ��Ӧ����̼��Ƴ�����ˮ������ʽ�ǣ�Ca��OH��2+CO2=CaCO3��+H20��
��5��������Ϣ��ʵ�����г��ü����Ȼ�泥�NH4Cl�����������ƹ�������ķ�����ȡ������NH3����ͬʱ�������Ȼ��ƺ�ˮ������֪����ȡ�����Ļ�ѧ����ʽ�ǣ�
2NH4Cl+Ca��OH��2
| ||
��6���ɵ�һ��ʵ��ͼ��֪�������쵽�������Թܵײ������������ϵ��³����Թܵģ�˵���������ܶȱȿ�����С����ˮ�������İ����У�Ȼ����ȥ��ñ����
���з�̪��ˮ�п�����ɫ��Ȫ��˵���Թ��ڵ�ѹǿ��С���Ұ�����ˮ��Ӧ���������Լ��ԣ��ɴ˿�֪����������ˮ��Ӧ���ɼ������ʣ�
�ʴ�Ϊ����1������©����ˮ�ۣ�
��2��2KMnO4
| ||
��3��������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��4��ʹ����Ũ���ᣨ�������ɣ���Ca��OH��2+CO2=CaCO3��+H20��
��5��2NH4Cl+Ca��OH��2
| ||
��6���������ܶȱȿ�����С�������𰸾��ɣ���
������������Ҫ�����˷���ʽ����д��������̼�����ʣ�װ���ص�ķ���������ʵ�������ܽ��������ʣ��ܺܺÿ���ѧ����֪ʶ�����պ�Ӧ�����������ڶ�ѧ��������������

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�
�����Ŀ
