��Ŀ����
��3�֣����ڷ��õ���������������ˮ�ͱ��ʡ�ʵ��������220 g���õĵ��������ƹ�����Ʒ��������Ʒ�и��ɷֵķֲ��Ǿ��ȵģ���ijʵ��С��Ӹ���Ʒ��ȡ��20 g�����Ⱥ�����е�ˮ�֣��õ�18.6 g���塣�����ù����м�������ij���ʯ��ˮ����ַ�Ӧ��õ�10 g���������������ݷ��������С��ͬѧ��ʣ�������������Ʒ�м�����һ�������������Ʒ�ĩ��ˮ����ַ�Ӧ���ˣ�ǡ�õõ���10%������������Һ�����������������ƺ�ˮ��������
74g��1426g
��������������ⷨһ����Ϊ 20 g ��Ʒ��ɺ�õ�18.6 ���壬����ʯ��ˮ��õ�10 ��������ʣ��200g��Ʒ�к��е�̼���ƺ��������ƹ��干�� 186 ���壬������ʯ�Һ�õ�̼���100 g��
����Ʒ��̼��������Ϊx�������������Ƶ�����Ϊy����Ӧ����NaOH������Ϊz��
Na2CO3 + Ca(OH)2 ="===" CaCO3�� + 2NaOH
106 74 100 80
x y 100 g z
106:100=x��100g 74:100=y��100g 100:80=100g��z
x=106g y=" 74g" z =80g
������Һ�����ʵ�����Ϊ186g �C 106 g + 80 g = 160g
������Һ��ˮ������Ϊ160g��10% �C 160g = 1440g
���� �����ˮ��Ϊ1440g �C��200g �C 186g��=" 1426g"
�𣺼����������������Ϊ74 g�������ˮ����Ϊ1426 g��
�ⷨ����
�⣺��20 g��Ʒ�к��е�̼��������Ϊ x
Na2CO3 + Ca(OH)2 ="===" Ca CO3�� + 2NaOH
106 100
x 10 g =
= 
x=" 10.6" g
���� ��Ʒ��̼���Ƶ���������Ϊ
�������Ƶ���������Ϊ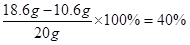
ˮ����������Ϊ1- 53% - 40% = 7%
������������Ƶ�����Ϊ y����Ӧ����NaOH������Ϊ z
Na2CO3 + Ca(OH)2 ==== Ca CO3�� + 2NaOH
106 74 80
(220-20) g��53% y z
106��[(220-20) g��53%]=74��y 106��[(220-20) g��53%]=80��z
y=" 74" g z =80g
������Һ�����ʵ�����Ϊ��220g �C 20g���� 40% + 80g = 160g
������Һ��ˮ������Ϊ160g��10% �C 160g = 1440g
���� �����ˮ��Ϊ1440g �C��220g �C 20g����7% = 1426g
�𣺼����������������Ϊ74 g�������ˮ����Ϊ1426 g��
���㣺���ݻ�ѧ����ʽ���㣻��Һ����������������
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢��
��Һ��������������= ��
��

 ��У����ϵ�д�
��У����ϵ�д�