��Ŀ����
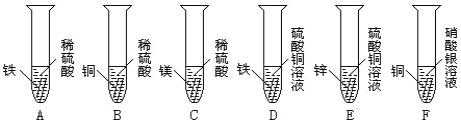
����Ŀ�� ��7�֣�����ͼ��ʾΪʵ�����г��������Ʊ�������������ռ�������ʵ��IJ�����������װʵ��װ��ʱ�����ظ�ѡ����������ijѧУ
������ѧʵ��̽���С���ͬѧ������������ɸ��Ե�̽��ʵ�顣
��1����һ���ͬѧ��ʯ��ʯ��ϡ����Ϊԭ�ϣ���ʵ�����Ʊ����ռ����﴿���Ķ�����̼���壬����Ҫ�����ʵ��װ�á�����������������װ�õ������ԡ�����ʾ���ӷ���������HCl������ñ���̼��������Һ���գ�
����ѡ����������˳��Ϊ________��_______��________��________����д���������ĸ����
��д��ʵ������ȡ������̼�ķ�Ӧ����ʽ_____________________________________��
�����鼯��ƿ�������Ƿ�Ϊ������̼�ľ��巽��________________________________
_________________________________________________________________________��
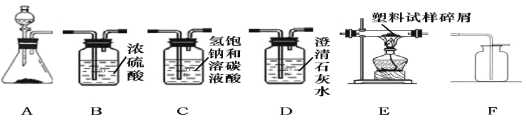
��2���ڶ����ͬѧ�Թ���������ҺΪԭ�ϣ�MnO2Ϊ�������Ʊ�����������ij���������ϵ����Ԫ�ؽ��з���̽����������ʾ����������һ����C��H��N����Ԫ�ء�
��ѡ��������A��B1��E��B2��D����ʯ�Ҹ���ܡ���˳�����ӣ���B1��B2ΪŨ����ϴ��ƿ����ʵ��ǰ����װ�������ԡ�ʹ������������м�ڴ������г��ȼ�գ��۲������ռ��й�ʵ�����ݣ����跢���Ļ�ѧ��Ӧ����ַ�Ӧ�����Իش���������
����A����������Ӧ�Ļ�ѧ����ʽ________________________________________��
����D�����������__________________________________________________��
������E�в������з��������������м����Ϊ3.5g�������������ȼ�պ������B2����������3.6g���������������HԪ�ص���������Ϊ________�����������һλС����
����װ����û����������B1������������ĸ�����������HԪ�ص�����������ʵ��ֵ�ȽϽ�_______(�ƫС����ƫ������һ�¡�֮һ)��
���𰸡��Ţ� ACBF �� CaCO3+2HCl CaCl2+H2O+CO2������������ʯ��ˮ����ʯ��ˮ����ǣ����ռ���������Ϊ������̼
�� �� 2H2O2 MnO2 2H2O+O2�� �� ����� �� 11.4% �� ƫ��
��������
�����������1����������Ʊ��У��ȳ����ٸ����ʯ��ʯ��ϡ����Ϊԭ����ȡ���ռ���������Ķ�����̼��Ӧ����ѡ���Һ��Ӧ��Aװ����Ϊ����װ�ã���̼ͨ��������Һ��ȥ�Ȼ������ʣ���ͨ��Ũ������ˮ���Ȼ���������ſ������ռ���ʯ��ʯ��ϡ���ᷴӦ���ɵ��Ƕ�����̼��ˮ���Ȼ��ƣ�CaCO3+2HCl CaCl2+H2O+CO2�������ݶ�����̼�����ʣ�������̼��ʹ�����ʯ��ˮ����ǣ��ʶ�����̼�ļ��鷽���ǣ�������ƿ�е��������ij����ʯ��ˮ����ʯ��ˮ����ǣ����ռ���������Ϊ������̼��
��2���Թ���������ҺΪԭ�ϣ�MnO2Ϊ�������Ʊ����������ɵ���ˮ��������2H2O2 MnO2 2H2O+O2������������ȼ�ջ����ɶ�����̼����D�еij���ʯ��ˮ�����ǣ�����B2����������3.6g�������ɵ�ˮ������Ϊ3.6g�����������غ㶨�ɻ�ѧ��Ӧǰ��Ԫ�ص����������֪��Ԫ�ص�����Ϊ��
3.6g��2��18 ��100%=0.4g����ô������������HԪ�ص���������Ϊ0.4��3.5g ��100%=11.4%����װ����û����������B1����ôͨ��������п��ܺ���ˮ���������²ⶨ��ˮ������ƫ�Ӷ�������Ԫ�ص���������ƫ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�