��Ŀ����
��6�֣��������ӹ���������ʶ���ʼ���ѧ��Ӧ�ı��ʡ�
�����·��Ż�ṹʾ��ͼ��ʾ�������У�����ԭ�ӵ��ǣ�����ţ���ͬ�����������������ӵ���������
��N2 ��NH4+ ��CO32-
��Hg �� ��
�� ��
��
����֪��ԭ�����������6�����ӣ���ѧ��Ӧ�У�ÿ����ԭ�ӵõ������������γ������ӡ�
�Ǵӷ��ӵĽǶȿ���ǽ�ڿ���ǽ�����ԭ������������ˮ�봿ˮ�ı���������������
�ȴ����ӵĽǶȿ������и��ֽⷴӦ��ʵ����������
�� BaCl2+H2SO4= BaSO4��+2HCl
�� BaCl2 + K2SO4 = BaSO4��+2KCl
�� Ba(OH)2+Na2SO4= BaSO4��+2NaOH
�� �ܢ� �ۢ� �� 2
�Ƿ����Dz����˶��� ��ˮ�������ַ��ӣ���ˮ��ֻ��һ�ַ���
�� Ba2+�ͽ��SO42-����BaSO4����
�������������������ڵ�������������ʾԭ�ӣ����������ڵ�����������ʾ�����ӣ�������С�ڵ�����������ʾ������
������������8�����ȶ��ṹ��
��3�����к��еķ��Ӳ����˶�����������ɢ��ʹ�����ŵ����㣮��ˮ�봿ˮ�ı��������Ƿ��ӵ����ͬ
�ȸ��ֽⷴӦ��ʵ�ʾ�����Һ�����Ӽ�ķ�Ӧ����Һ�����Ӽ����γɳ����������ˮ�����ֽⷴӦ���ܷ�����

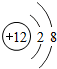 ��
�� ��
��
 ��
�� ��
��
 ��
�� ��
��
 ��
�� ��
��
 ��
�� ��
��