��Ŀ����
СԶͨ����������֪�����������Ҫ�ɷ֣�����һ��������̼��Ʒ�ĩ��ʳ�ε���С�ձ��У�Ȼ������������ͣ���ʪ��������ζ�����㾫�ȣ�������Ⱥ��Ƶ����࣮��1�������� ������������
��2��СԶ�ⶨ�������༰���������Ʒ��pH����¼���£�
| �� �� | �������� | ����� | ���۾� |
| pH | 8 | 2 | 12 |
��3�������е�̼��Ʒ�ĩ��Ħ��������߽��Ч��������̼����ǽ�������̼ͨ��ʯ�ҽ��Ƶõģ���д����ʯ��ʯ��ˮΪԭ����ȡ����̼��ƵĻ�ѧ����ʽ�� ��
���𰸡��������������ʵķ���������ʸ�������Ƿ�һ�ķ�Ϊ�������һ��֣��ͻ������ֻ��������ϣ�����Һ���������PHֵ�Ĺ�ϵΪ��PH=7Ϊ���ԣ�PH��7Ϊ���ԣ�PH��7Ϊ���ԣ������ָʾ����ɫʯ������������������⣨3��Ϊʯ��ʯ����ʯ�ҡ���ʯ�Ҽ��ת����
����⣺��1������������Ʒ���һ��������̼��Ʒ�ĩ��ʳ���У������������ͣ���ʪ��������ζ�����㾫�ȣ�������Ⱥ��Ƶ����࣮�ʴ�Ϊ�������
��2����������PH��7Ϊ���ԣ������PH��7Ϊ���ԣ�����ɫʯ������������������ʴ�Ϊ�����ԣ���ɫ
��3��ʯ��ʯ��ˮΪԭ����ȡ̼��Ƶķ�ӦΪ��CaCO3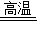 CaO+CO2����CaO+H2O=Ca��OH��2��Ca��OH��2+CO2=CaCO3��+H2O
CaO+CO2����CaO+H2O=Ca��OH��2��Ca��OH��2+CO2=CaCO3��+H2O
���������⿼�������ʵķ��ࡢ��Һ����Լ�������ʯ��ʯ����ʯ�ҡ���ʯ�Ҽ��ת����ϵ�����û���֪ʶ���⣮
����⣺��1������������Ʒ���һ��������̼��Ʒ�ĩ��ʳ���У������������ͣ���ʪ��������ζ�����㾫�ȣ�������Ⱥ��Ƶ����࣮�ʴ�Ϊ�������
��2����������PH��7Ϊ���ԣ������PH��7Ϊ���ԣ�����ɫʯ������������������ʴ�Ϊ�����ԣ���ɫ
��3��ʯ��ʯ��ˮΪԭ����ȡ̼��Ƶķ�ӦΪ��CaCO3
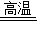 CaO+CO2����CaO+H2O=Ca��OH��2��Ca��OH��2+CO2=CaCO3��+H2O
CaO+CO2����CaO+H2O=Ca��OH��2��Ca��OH��2+CO2=CaCO3��+H2O���������⿼�������ʵķ��ࡢ��Һ����Լ�������ʯ��ʯ����ʯ�ҡ���ʯ�Ҽ��ת����ϵ�����û���֪ʶ���⣮

��ϰ��ϵ�д�
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ
СԶͨ����������֪�����������Ҫ�ɷ֣�����һ��������̼��Ʒ�ĩ��ʳ�ε���С�ձ��У�Ȼ������������ͣ���ʪ��������ζ�����㾫�ȣ�������Ⱥ��Ƶ����࣮
��1�������� ������������
��2��СԶ�ⶨ�������༰���������Ʒ��pH����¼���£�
���������� �������ԡ����ԡ����ԣ���������ʹ��ɫʯ����Һ��� ��
��3�������е�̼��Ʒ�ĩ��Ħ��������߽��Ч��������̼����ǽ�������̼ͨ��ʯ�ҽ��Ƶõģ���д����ʯ��ʯ��ˮΪԭ����ȡ����̼��ƵĻ�ѧ����ʽ�� ��
��1��������
��2��СԶ�ⶨ�������༰���������Ʒ��pH����¼���£�
| �� �� | �������� | ����� | ���۾� |
| pH | 8 | 2 | 12 |
��3�������е�̼��Ʒ�ĩ��Ħ��������߽��Ч��������̼����ǽ�������̼ͨ��ʯ�ҽ��Ƶõģ���д����ʯ��ʯ��ˮΪԭ����ȡ����̼��ƵĻ�ѧ����ʽ��
СԶͨ����������֪�����������Ҫ�ɷ֣�����һ��������̼��Ʒ�
ĩ��ʳ�ε���С�ձ��У�Ȼ������������ͣ���ʪ��������ζ�����㾫�ȣ�������Ⱥ��Ƶ����࣮
��1�������� ����������
��2��СԶ�ⶨ�������༰���������Ʒ��pH����¼���£�
���������� �������ԡ����ԡ����ԣ���������ʹ��ɫʯ����Һ��� ��
��3�������е�̼��Ʒ�ĩ��Ħ��������߽��Ч��������̼����ǽ�������̼
ͨ��ʯ�ҽ��Ƶõģ���д����ʯ��ʯ��ˮΪԭ����ȡ����̼��ƵĻ�ѧ����ʽ�� ��
ĩ��ʳ�ε���С�ձ��У�Ȼ������������ͣ���ʪ��������ζ�����㾫�ȣ�������Ⱥ��Ƶ����࣮
��1��������
��2��СԶ�ⶨ�������༰���������Ʒ��pH����¼���£�
| ���� | �������� | ����� | ���۾� |
| pH | 8 | 2 | 12 |
��3�������е�̼��Ʒ�ĩ��Ħ��������߽��Ч��������̼����ǽ�������̼
ͨ��ʯ�ҽ��Ƶõģ���д����ʯ��ʯ��ˮΪԭ����ȡ����̼��ƵĻ�ѧ����ʽ��
СԶͨ����������֪�����������Ҫ�ɷ֡�����һ��������̼��Ʒ�
ĩ��ʳ�ε���С�ձ��У�Ȼ������������ͣ���ʪ��������ζ�����㾫�ȣ�������Ⱥ��Ƶ����ࡣ
�� ������__________________����������
�� СԶ�ⶨ�������༰���������Ʒ��pH����¼���£�
| �� �� | �������� | ����� | ���۾� |
| pH | 8 | 2 | 12 |
����������____________(�����ԡ����ԡ�����)��������ʹ��ɫʯ����Һ���_______________��
�� �����е�̼��Ʒ�ĩ��Ħ��������߽��Ч��������̼����ǽ�������̼
ͨ��ʯ�ҽ��Ƶõģ���д����ʯ��ʯ��ˮΪԭ����ȡ����̼��ƵĻ�ѧ����ʽ��___________________________________________________��
