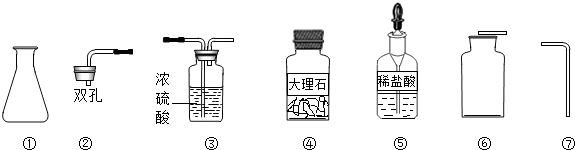
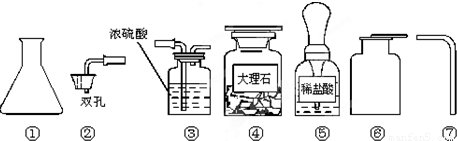
��Ŀ����
Ϊ��ȡ���ռ�һƿ�����CO2���壬ij�����о�С����ʵ���ʦ��ȡ������������ҩƷ��
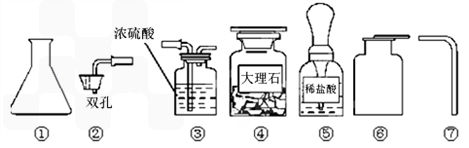
��1��д��������������ƣ��� ���� ��Ҫ��ɸ�ʵ�飬���ǻ�������ʵ����ʦ��ȡ�����������Ӻ� ��
��2��ʵ������ȡʱCO2������Ӧ�Ļ�ѧ����ʽΪ ��
��3���ռ�CO2ʱ��������μ�����CO2�ռ����� ��
��4��ʵ������ȡ����Ҫѡ���ʵ���Ӧ�����֪��ʵ������ȡ����CO2���岻����Ũ���ᣬ��Ϊ ��Ҳ������ϡ���ᣬ��Ϊ ��
��5�����Ƕ�ʵ������ȡCO2��ʵ������չ���˽�һ����̽�������������ĸ��Ա�ʵ�飺
��ȡm g��״����ʯ��������������������Ϊ5%�����ᷴӦ��
��ȡm g��״����ʯ��������������������Ϊ10%�����ᷴӦ��
ʵ�����������ݵĿ���˳��Ϊ�ڣ��٣�
ʵ����ۣ�Ӱ�����ʯ�����ᷴӦ������������ ��
��2��ʵ������ȡʱCO2������Ӧ�Ļ�ѧ����ʽΪ ��
��3���ռ�CO2ʱ��������μ�����CO2�ռ����� ��
��4��ʵ������ȡ����Ҫѡ���ʵ���Ӧ�����֪��ʵ������ȡ����CO2���岻����Ũ���ᣬ��Ϊ ��Ҳ������ϡ���ᣬ��Ϊ ��
��5�����Ƕ�ʵ������ȡCO2��ʵ������չ���˽�һ����̽�������������ĸ��Ա�ʵ�飺
��ȡm g��״����ʯ��������������������Ϊ5%�����ᷴӦ��
��ȡm g��״����ʯ��������������������Ϊ10%�����ᷴӦ��
ʵ�����������ݵĿ���˳��Ϊ�ڣ��٣�
ʵ����ۣ�Ӱ�����ʯ�����ᷴӦ������������ ��
��1����ƿ������ƿ������©��
��2��CaCO3+2HCl=CaCl2+H2O+CO2��
��3����һ��ȼ�ŵ�ľ��ƽ���ڼ���ƿ�ڣ����ľ��Ϩ�𣬾�֤������
��4��Ũ�����ӷ����ᵼ���ռ����Ķ�����̼�л����Ȼ������壻��Ӧ���������������ˮ�����谭��Ӧ�Ľ��У�
��5���������������
��2��CaCO3+2HCl=CaCl2+H2O+CO2��
��3����һ��ȼ�ŵ�ľ��ƽ���ڼ���ƿ�ڣ����ľ��Ϩ�𣬾�֤������
��4��Ũ�����ӷ����ᵼ���ռ����Ķ�����̼�л����Ȼ������壻��Ӧ���������������ˮ�����谭��Ӧ�Ľ��У�
��5���������������

��ϰ��ϵ�д�
�����Ŀ