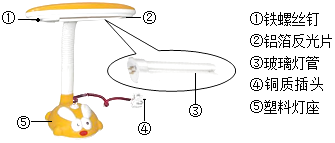
��Ŀ����
����Ŀ��Һ��ʯ��������ͼ������Ҫ�ļ���ȼ�ϣ� 
��1��500L ��Һ�����ܱ�ѹ���� 25L �ĸ�ƿ�У�����Ϊ ��
��2��Һ��ʯ������ʯ�ͻ����ĸ���Ʒ��ʯ�͡���Ȼ����úͳ��Ϊ ��ʯȼ�ϣ�������Ȼ����ȫȼ�յĻ�ѧ����ʽΪ ��
��3��Һ��ʯ�����ж��飨C4H10���ĺ�����ߣ����ڶ��������˵���У���ȷ���� ������ĸ��ţ���
A.���������������
B.������̼Ԫ����������Ϊ 25%
C.������̼ԭ�Ӻ���ԭ�ӹ���
D.������п�ȼ�ԣ�
���𰸡�
��1������֮���м��
��2��CH4+2O2 ![]() CO2+2H2O
CO2+2H2O
��3��AD
���������⣺��1�����ڷ��Ӽ��м��������ѹʱ�����С�����ԣ�500L��Һ�����ܱ�ѹ����25L�ĸ�ƿ�У���2����Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪ��CH4+2O2 ![]() CO2+2H2O����3��A���л���������ָ��CԪ�صĻ�������ݶ���Ļ�ѧʽ��C4H10 �� ������C��H����Ԫ����ɵĻ���������л�������ʶԣ� B��������̼Ԫ����������Ϊ
CO2+2H2O����3��A���л���������ָ��CԪ�صĻ�������ݶ���Ļ�ѧʽ��C4H10 �� ������C��H����Ԫ����ɵĻ���������л�������ʶԣ� B��������̼Ԫ����������Ϊ ![]() ��82.8%���ʴ���
��82.8%���ʴ���
C�����ݶ���Ļ�ѧʽ��C4H10 �� �������4��Cԭ�Ӻ�10��Hԭ�������ɣ������Ƕ�����̼ԭ�Ӻ���ԭ�ӹ��ɣ��ʴ���
D��������п�ȼ�ԣ�������ȼ�ϣ��ʶԣ�
���Դ��ǣ���1������֮���м������2��CH4+2O2 ![]() CO2+2H2O����3��AD��
CO2+2H2O����3��AD��
�����㾫����������Ҫ��������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�ͳ���ȼ�ϵ�ʹ������Ի�����Ӱ������֪ʶ�㣬��Ҫ����ע�⣺a����ƽ b������ c�����ţ�úȼ���ŷŵ���Ⱦ�SO2��NO2���������꣩��CO���̳��ȣ�ʯ��ȼ������β������Ⱦ�CO��δȼ�յ�̼�⻯��������������Ǧ��������̳�����Ȼ���ǽ�������Դ������ȷ�����⣮

����Ŀ����ȤС���ͬѧ���֣�������ͼװ�ã����������ʵ�飬����ش��������⣺ 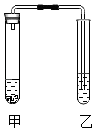
��1����ʵ�顿
1 | 2 | 3 | |
�ף�ҩƷ�� | Ũ��ˮ | ̼���ơ�ϡ���� | ˮ���������ƹ��� |
�ң�ҩƷ�� | ��ɫ��̪��Һ | ����ʯ��ˮ | ˮ |
���� | �ҵ��ܿ�������ð���� | �ҵ��ܿ�������ð�� | |
���ۺͽ��� | ���Ӿ��е�һ�������� | д�����е�һ����ѧ����ʽ�� | ���ָ������ԭ���� |
��2�������������ۡ� ��ʵ��3�н��������ƹ��廻�� �� ԭ��һ����Ҳ�������ͬ������
�ڷ�Ӧֹͣ��ʵ��2�мס������Թܷ�Һ����һ�𣬲�������ij�������Ӧֹͣ��ʵ��2���Թ���Һ�е��������� ��