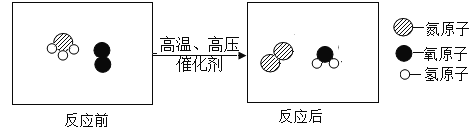
��Ŀ����
����Ŀ�����������ĺϽ𣬻�ѧ��ȤС���ͬѧΪ�ⶨij������Ʒ������������������������ʵ�飺��ȡ������Ʒ5.8g�����ձ��У���μ���ϡ���ᣬ����ϡ�����������ų������������ϵ��ͼ��ʾ��������Ʒ�е����ʲ���ϡ���ᷴӦ��Ҳ������ˮ�����ش��������⣺
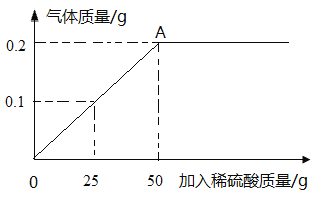
��1�����μ�ϡ��������Ϊ ʱ����Ӧǡ����ȫ����ʱ�������������� ��
��2������������Ʒ�����������������������0.1%����ͬ����
��3����������ϡ���������ʵ���������������ܰ��ʾ��Fe+H2SO4=FeSO4+H2����
���𰸡���1��50g 0.2g ��2��98.3% ��3��19.6%
��������
�����������1�����ͼ�������Ŀץס������һ���ơ��������ȿ�����ᡢ���������ʾ������Ȼ�����ʼ�㣨��Ӧ��ʼ����ת�۵㣨��Ӧ���������յ㣬�ʸ���ͼʾ�����μ�ϡ��������Ϊ50gʱ����Ӧǡ����ȫ����ʱ��������������0.2g
��2�����ݻ�ѧ����ʽ��Fe+H2SO4 =FeSO4 +H2����H2��Fe��H2SO4��������ϵ�����ȷֱ����Fe��H2SO4���������ٽ�һ������������Ʒ���������������Լ�����ϡ���������ʵ���������
�⣺��Fe������Ϊx��H2SO4������Ϊy
Fe+H2SO4 =FeSO4 +H2��
56 98 2
x y 0.2g
(2)56��2=x��0.2g x=5.6g
������Ʒ��������������=5.6g/5.8g��100%=98.3%
(3) 98��2=y��0.2g y=9.8g
����ϡ���������ʵ���������=9.8g/50g��100%=19.6%