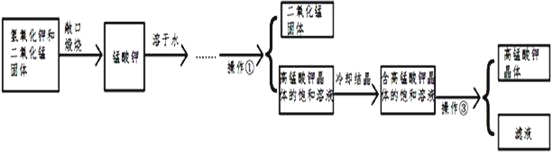
��Ŀ����
����Ŀ�������NaCl��KNO3�IJ����ܽ�ȣ���ش��������⣺
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 |
NaCl���ܽ��/g | 35.8 | 36.0 | 36.63 | 36.6 | 37.0 | 37.3 |
KNO3���ܽ��/g | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110.0 |
��1��NaCl��KNO3���ܽ�����¶ȱ仯Ӱ������ ��
��2��20��ʱ���Ȼ��Ƶ��ܽ��Ϊ ��ȡ20g�Ȼ��Ʒ���50gˮ���ֽ��裬�ɵõ���Һ g��
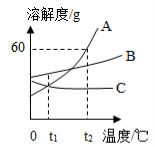
��3����ͼ��ʾ���в�����ÿ��������ܽ⣩���ɵõ�������Һ���� ������ĸ���������������������� ������ĸ����

���𰸡�
��1��KNO3
��2��36.0g 68
��3��A B
��������
���������
��1���ɱ��е����ݿ�֪��NaCl��KNO3���ܽ�����¶ȱ仯Ӱ������KNO3��
��2��20��ʱ���Ȼ��Ƶ��ܽ��Ϊ36.0g�����ܽ�ȵĺ����֪��ȡ20g�Ȼ��Ʒ���50gˮ���ֽ��裬ֻ���ܽ�18g���ɵõ���Һ��������50g+18g=68g��
��3���ɱ��е����ݿ�֪��20��ʱ������ص��ܽ����31.6g����20��ʱ��40g������ؼ��뵽100g��ˮ��ֻ���ܽ�31.6g���γɵ���ҺA�DZ��ͺ���Һ������Һ����ɿ�֪������ҺB�У�100g��ˮ���ܽ������ص�������࣬�����������������

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�����Ŀ�������Ԫ�����ڱ���һ���֣�
�� ���� | IA | 0 | ||||||
1 | 1 H 1.008 | ��A | ��A | ��A | V A | ��A | ��A | 2 He 4.003 |
2 | 3 Li 6.941 | 4 Be 9.012 | 5 B 10.8l | 6 C 12.01 | 7 N 14.0l | 8 O 16.00 | 9 F 19.00 | 10 Ne 20.18 |
3 | 11 Na 22.99 | 12 Mg 24.31 | 13 Al 26.98 | 14 Si 28.09 | 15 P 30.97 | 16 S 32.06 | 17 Cl 35.45 | 18 Ar 39.95 |
��1��ԭ��������12��Ԫ�ط���Ϊ ��BeԪ�ص����ԭ������Ϊ ��
��2�����ԭ������Ϊ22.99��Ԫ���� ����������ǽ�������Ԫ�أ�Ne�Ļ�ѧ���ʱȽ� ����ȶ������ȶ�������
��3����ͼ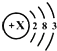 ΪijԪ�ص�ԭ�ӽṹʾ��ͼ����Ԫ��λ�����ڱ��е� ���ڣ�
ΪijԪ�ص�ԭ�ӽṹʾ��ͼ����Ԫ��λ�����ڱ��е� ���ڣ�
X= ����һ������ �����ӣ�������� �����ӣ���ԭ���ڻ�ѧ��Ӧ������ ���ʧȥ���õ��������ӣ���ԭ�ӵ�Ԫ�ط����� ��Ԫ�������� ������ �������Ԫ�ء��ǽ���Ԫ�ء�����