��Ŀ����
��2008?���ݣ�Ϊ�ⶨNaCl��Na2CO3����������ɣ�ij��ѧ����С��ȡ29g�û�������ձ��У�����μ���ϡ���ᣨÿ��50g����ʵ�������������±���| ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� |
| ����ϡ��������� | 50g | 50g | 50g | 50g | 50g |
| �ձ����ձ������ʵ������� | 159.1g | 206.9g | 254.7g | 302.5g | 352.5g |
��ʵ����Ϻ���Һ��NaCl���������������ϼ�����������һλС������
���𰸡�������NaCl��Na2CO3���������еμ����ᣬ̼���������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��������̼�ų�ʹ��Ӧǰ����������С����ȫ��Ӧ��������ҺΪ�Ȼ�����Һ�����������Ȼ���Ϊԭ��Ϲ��������Ȼ��������뷴Ӧ�����Ȼ�������֮�ͣ�
�ڶ��η�Ӧǰ���ձ����ձ������ʵ���������=159.1g+50g-206.9g=2.2g��
�����η�Ӧǰ���ձ����ձ������ʵ���������=206.9g+50g-254.7g=2.2g��
���Ĵη�Ӧǰ���ձ����ձ������ʵ���������=254.7g+50g-302.5g=2.2g��
����η�Ӧǰ���ձ����ձ������ʵ���������=302.5g+50g-352.5g=0��˵�����Ĵλ�����е�̼��������ȫ��Ӧ��ǰ�Ĵη�Ӧÿ�η�Ӧ�ų�2.2g������̼���Ĵη�Ӧ���ų�������̼������=��159.1g+50g-206.9g��×4=8.8g��
���ݷ�Ӧ�Ļ�ѧ����ʽ�����÷�Ӧ���ɶ�����̼����������ɼ��㷴Ӧ����̼���Ƶ������������Ȼ��Ƶ�������
����⣺�����⣬���Ĵ�ʵ��������ȫ��Ӧ��ʵ����Ϻ����ɶ�����̼������=��159.1g+50g-206.9g��×4=8.8g
��ԭ�������̼���Ƶ�����Ϊx������ϡ���ᷴӦ���ɵ��Ȼ��Ƶ�����Ϊy
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 117 44
x y 8.8g
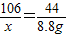 x=21.2g
x=21.2g
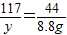 y=23.4g
y=23.4g
��1��ԭ�������Na2CO3����������=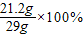 =73.1%
=73.1%
��2��ʵ����Ϻ���Һ��NaCl����������=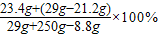 =11.5%
=11.5%
�𣺢�ԭ�������Na2CO3����������Ϊ73.1%��
��ʵ����Ϻ���Һ��NaCl����������Ϊ11.5%��
���������������غ㶨�ɣ���Ӧ��������Һ������=������������+�������ϡ���������-�ų�������̼��������
�ڶ��η�Ӧǰ���ձ����ձ������ʵ���������=159.1g+50g-206.9g=2.2g��
�����η�Ӧǰ���ձ����ձ������ʵ���������=206.9g+50g-254.7g=2.2g��
���Ĵη�Ӧǰ���ձ����ձ������ʵ���������=254.7g+50g-302.5g=2.2g��
����η�Ӧǰ���ձ����ձ������ʵ���������=302.5g+50g-352.5g=0��˵�����Ĵλ�����е�̼��������ȫ��Ӧ��ǰ�Ĵη�Ӧÿ�η�Ӧ�ų�2.2g������̼���Ĵη�Ӧ���ų�������̼������=��159.1g+50g-206.9g��×4=8.8g��
���ݷ�Ӧ�Ļ�ѧ����ʽ�����÷�Ӧ���ɶ�����̼����������ɼ��㷴Ӧ����̼���Ƶ������������Ȼ��Ƶ�������
����⣺�����⣬���Ĵ�ʵ��������ȫ��Ӧ��ʵ����Ϻ����ɶ�����̼������=��159.1g+50g-206.9g��×4=8.8g
��ԭ�������̼���Ƶ�����Ϊx������ϡ���ᷴӦ���ɵ��Ȼ��Ƶ�����Ϊy
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 117 44
x y 8.8g
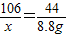 x=21.2g
x=21.2g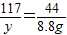 y=23.4g
y=23.4g��1��ԭ�������Na2CO3����������=
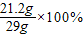 =73.1%
=73.1%��2��ʵ����Ϻ���Һ��NaCl����������=
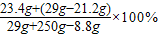 =11.5%
=11.5%�𣺢�ԭ�������Na2CO3����������Ϊ73.1%��
��ʵ����Ϻ���Һ��NaCl����������Ϊ11.5%��
���������������غ㶨�ɣ���Ӧ��������Һ������=������������+�������ϡ���������-�ų�������̼��������

��ϰ��ϵ�д�
�����Ŀ