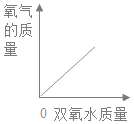
��Ŀ����
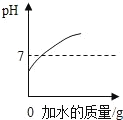
����Ŀ��ij�о���ѧϰС�鵽��������һ�����棬��װ���ϱ�����Ҫ�ɷ���̼���ƣ��������������Ȼ��ơ�����ȤС��Ϊ�о���ɷ֣���ȡ��Ʒ25.0g���������Ƴ���Һ������������μ���100g��������������Ϊ14.6%��ϡ������ǡ����ȫ��Ӧ����Ӧ���ɶ�����̼���������������ϡ����������ϵ��ͼ���Իش��������⣺
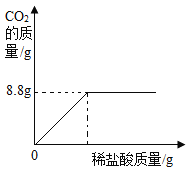
��1����Ʒ��ַ�Ӧ������CO2������Ϊ_____g��
��2��ԭ������Na2CO3�����������Ƕ��٣�_____��Na-23 Cl-35.5 H-1 C-12 O-16��
���𰸡�8.8 84.8%
��������
��1����ͼ���֪����Ʒ��ַ�Ӧ������CO2������Ϊ8.8g��
��2����ԭ������̼���Ƶ�����Ϊx
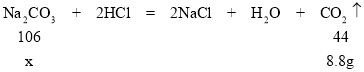
![]()
��ã�x=21.2g
ԭ������Na2CO3������������Na2CO3%=![]() ��
��

��ϰ��ϵ�д�
�����Ŀ