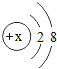ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΩΤ―ßΧΫΨΩΘΚ
![]() Β―ιΟϊ≥ΤΘΚΨΤΨΪΒΤ»Φ…’ΒΡΧΫΨΩΘΜ
Β―ιΟϊ≥ΤΘΚΨΤΨΪΒΤ»Φ…’ΒΡΧΫΨΩΘΜ
![]() Β―ιΡΩΒΡΘΚ
Β―ιΡΩΒΡΘΚ
ΔΌ»œ Ε“«ΤςΨΤΨΪΒΤΦΑΤδ”ΟΆΨΓΔ Ι”ΟΖΫΖ®Θ°________Θ°
Δέ―ßœΑΙέ≤λ Β―ιΒΡΖΫΖ®ΦΑ Β―ι±®ΗφΒΡΧν–¥Θ°
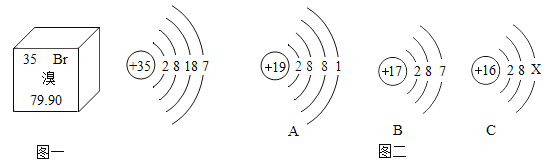
![]() Β―ι“«ΤςΘΚ________
Β―ι“«ΤςΘΚ________
![]() Β―ι“©ΤΖΦΑΤδΥϊ≤ΡΝœΘΚ________Θ°
Β―ι“©ΤΖΦΑΤδΥϊ≤ΡΝœΘΚ________Θ°
![]() Χν±μΗώΘΚ
Χν±μΗώΘΚ
Β―ι≤Ϋ÷η | œ÷œσ | Ζ÷Έω |
»Γ…ΌΝΩΨΤΨΪΒΙ»κ…’±≠÷–Φ” ΝΩΥ° | ________ | ΨΤΨΪ________»ήΫβ”ΎΥ°Θ®ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±Θ© |
¥ρΩΣΨΤΨΪΒΤΘ§ΫΪΒΤΟ±Ζ≈‘ΎΉά…œΘ§”ΟΜπ≤ώΒψ»ΦΨΤΨΪΒΤ | Μπ―φΖ÷ ________≤ψ | ________ |
‘ΎΜπ―φ…œΖΫ ________ | ”Ο ÷Οΰ–Γ…’±≠________…’±≠ΡΎ±Ύ ________ | ”–________Ζ≈≥ωΘ§”– ________…ζ≥… |
»Γœ¬________ΒΙ»κ________ | ________ | ”–________…ζ≥… |
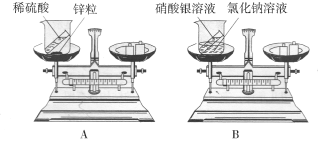
![]() Ϋα¬έΘΚ________Θ°
Ϋα¬έΘΚ________Θ°
ΓΨ¥πΑΗΓΩΧΫΨΩΨΤΨΪ»Φ…’ΒΡ≤ζΈοΨΤΨΪΒΤΓΔ…’±≠≥Έ«ε ·Μ“Υ°ΓΔΜπ≤ώΨΤΨΪ”κΥ°ΜΞ»ήΘ§«“≤ΜΖ÷≤ψΡή»ΐΆβ―φΈ¬Ε»ΉνΗΏ’÷“ΜΗ…ΕχάδΒΡ…’±≠ΖΔΧΧ”–Υ°ΈμΓΔ»»ΝΩΖ≈≥ωΥ°…’±≠≥Έ«ε ·Μ“Υ°≥Έ«ε ·Μ“Υ°±δΜκΉ«Εΰ―θΜ·ΧΦΨΤΨΪ»Φ…’…ζ≥…Εΰ―θΜ·ΧΦΚΆΥ°Θ§Ζ≈≥ω»»ΝΩ
ΓΨΫβΈωΓΩ
Θ®2Θ©ΔΎ Β―ι±®ΗφΒΡΡΎ»ί÷–―ι÷ΛΝΥΕΰ―θΜ·ΧΦΚΆΥ°ΒΡ…ζ≥…Θ§Υυ“‘Ω…“‘ΆΤ≤β≥ω Β―ιΡΩΒΡ «ΧΫΨΩΨΤΨΪ»Φ…’ΒΡ≤ζΈοΘΜ
Θ®3Θ© Β―ι“«Τς”ΟΒΫΨΤΨΪΒΤΓΔ…’±≠ΘΜ
Θ®4Θ© Β―ι±®ΗφΒΡΡΎ»ί÷–―ι÷ΛΝΥΕΰ―θΜ·ΧΦΚΆΥ°ΒΡ…ζ≥…Θ§Ι Φλ―ιΕΰ―θΜ·ΧΦ–η“Σ≥Έ«ε ·Μ“Υ°ΘΜΧΫΨΩΨΤΨΪ»Φ…’ΒΡ≤ζΈο–η“ΣΫΪΨΤΨΪΒΤΒψ»ΦΘ§Ι –η“ΣΜπ≤ώΘΜ
Θ®5Θ©
≤Ϋ÷η“ΜΘΚ»Γ…ΌΝΩΨΤΨΪΒΙ»κ…’±≠÷–Φ” ΝΩΥ°Θ§Μα–Έ≥…Ψυ“ΜΓΔΈ»Ε®ΒΡΜλΚœΈοΘ§ΨΤΨΪ“Ή»ή”ΎΥ°ΘΜ
≤Ϋ÷ηΕΰΘΚ¥ρΩΣΨΤΨΪΒΤΘ§ΫΪΒΤΟ±Ζ≈‘ΎΉά…œΘ§”ΟΜπ≤ώΒψ»ΦΨΤΨΪΒΤΘ§Μπ―φΖ÷»ΐ≤ψΘ§Άβ―φΈ¬Ε»ΉνΗΏΘΜ
≤Ϋ÷η»ΐΘΚ‘ΎΜπ―φ…œΖΫ’÷“ΜΗ…ΕχάδΒΡ…’±≠Θ§”Ο ÷Οΰ…’±≠Άβ±ΎΖΔΧΧΘ§ΥΒΟςΨΤΨΪ»Φ…’Ζ≈≥ω»»ΝΩΘ§…’±≠ΡΎ±Ύ”–Υ°ΈμΘ§ΥΒΟς”–Υ°…ζ≥…ΘΜ
≤Ϋ÷ηΥΡΘΚ»Γœ¬…’±≠Θ§ΫΪΤδ―ΗΥΌΒΙΉΣΘ§Φ”»κ≥Έ«ε ·Μ“Υ°±δΜκΉ«Θ§ΥΒΟς”–Εΰ―θΜ·ΧΦ…ζ≥…ΘΜ
Θ®6Θ©ΗυΨί Β―ι±®ΗφΒΡΡΎ»ίΩ…÷ΣΘ§ΒΟΒΫΒΡΫα¬έΈΣΨΤΨΪ»Φ…’…ζ≥…Εΰ―θΜ·ΧΦΚΆΥ°Θ§Ζ≈≥ω»»ΝΩΓΘ

ΓΨΧβΡΩΓΩΡΩ«Α»Ϊ άΫγΒΡΡχ(Ni)œϊΖ―ΝΩΫω¥Έ”ΎΆ≠ΓΔ¬ΝΓΔ«ΠΓΔ–ΩΘ§Ψ””–…ΪΫπ τΒΎΈεΈΜΓΘΡχ≥Θ”Ο”ΎΗς÷÷ΗΏΙβ‘σΉΑ ΈΤαΚΆΥήΝœ…ζ≤ζΘ§“≤≥Θ”ΟΉς¥ΏΜ·ΦΝΓΘΩ…“‘ΥΒΡχ––“ΒΖΔ’Ι‘Χ≤ΊΉ≈Ψό¥σΒΡ«±ΝΠΓΘ
Δώ÷Τ±Η≤ίΥαΡχ
ΙΛ“Β…œΩ…άϊ”ΟΚ§ΡχΚœΫπΖœΝœ(≥ΐΡχΆβΘ§ΜΙΚ§”–FeΓΔCuΓΔCaΓΔMgΓΔCΒ»‘”÷ )÷Τ»Γ≤ίΥαΡχ(NiC2O4)ΓΘ
ΗυΨίœ¬Ν–ΙΛ“’Νς≥ΧΆΦΜΊ¥πΈ ΧβΘΚ
Θ®Ή ΝœΩ®Τ§1Θ©
(1)Ιΐ―θΜ·«β(H2O2)ΥΉ≥ΤΥΪ―θΥ°Θ§ «“Μ÷÷“ΚΧεΘ§ ή»»“ΉΖ÷ΫβΘ§Ω…”Ο”Ύ Β―ι “÷Τ»Γ―θΤχΘΜΙΐ―θΜ·«βΨΏ”–«Ω―θΜ·–‘Θ§Υυ“‘≥ΘΉςΈΣ―θΜ·ΦΝΓΔΤ·ΑΉΦΝΚΆœϊΕΨΦΝΓΘ
(2)―θΜ·Ζ¥”ΠΩ…“‘¥”‘ΣΥΊΜ·ΚœΦέ…ΐΫΒΒΡΫ«Ε»Ϋχ––Έο÷ ΥυΚ§‘ΣΥΊΜ·ΚœΦέ…ΐΗΏΒΡΖ¥”ΠΨΆ «―θΜ·Ζ¥”ΠΓΘ
(3)Ϋπ τΡχΒΡΜ·―ß–‘÷ άύΥΤ”ΎΧζΘ§”κ―ΈΥαΖ¥”ΠΡή…ζ≥…¬»Μ·Ρχ(NiCl2)ΓΘ
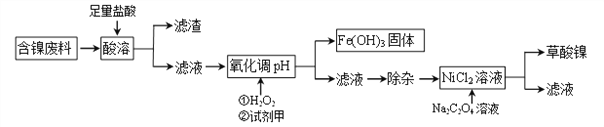
(1)ΓΑΥα»ήΓ±÷°ΚσΒΡ¬Υ‘ϋ÷–Κ§”–ΒΡΫπ τ «_______________ΓΘ
(2)–¥≥ωΥα»ήΙΐ≥Χ÷–Ni”κ―ΈΥαΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_________________________ΓΘ
(3)Φ”H2O2 ±Θ§Έ¬Ε»≤ΜΡήΧΪΗΏΘ§Τδ‘≠“ρ «_______________________ΓΘ»τH2O2‘ΎΓΑΥα»ήΓ±≤Ϋ÷ηΦ¥”κ―ΈΥαΆ§ ±Φ”»κΘ§‘ρ”κ‘≠ΖΫΑΗœύ±»¬Υ“Κ÷–Μα‘ωΦ”ΒΡΫπ τάκΉ” «Cu2+ΚΆ_______(–¥άκΉ”ΖϊΚ≈)ΓΘ
(4)“―÷ΣNa2C2O4»ή“ΚΦ”»κNiCl2»ή“ΚΖΔ…ζΗ¥Ζ÷ΫβΖ¥”ΠΘ§–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ____________ΓΘ
Δρ÷Τ±ΗΡχΖέ
ΙΛ“Β”ΟΒγΫβΡχ“Κ(÷ς“ΣΚ§NiSO4)÷Τ±ΗΦν ΫΧΦΥαΡχΨßΧεxNiCO3yNi(OH)2zH2OΘ§≤Δάϊ”ΟΤδ÷Τ±ΗΡχΖέΘ§Ιΐ≥Χ»γœ¬ΘΚ
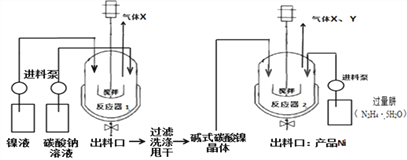
(5)Ζ¥”ΠΤς1÷–ΒΡ“ΜΗω÷Ί“ΣΖ¥”ΠΈΣ3NiSO4+3Na2CO3+2H2O ![]() NiCO32Ni(OH)2+3Na2SO4+2XΘ§XΒΡΜ·―ß ΫΈΣ_________ΓΘ
NiCO32Ni(OH)2+3Na2SO4+2XΘ§XΒΡΜ·―ß ΫΈΣ_________ΓΘ
(6)Ζ¥”ΠΤς1ΒΡ≥ωΝœΩΎΜώΒΟΒΡΙΧΧεœ¥Β” ±Θ§–η”Ο¥ΩΥ°œ¥Β”Θ§Ω…“‘”Οά¥Φλ―ιΙΧΧε“―œ¥Β”Η…ΨΜΒΡ ‘ΦΝ «____________ΓΘ
(7)Ζ¥”ΠΤς2÷–≤ζ…ζΒΡΤχΧεY «Ω’Τχ÷–Κ§ΝΩΉνΕύΒΡΤχΧεΘ§ΗΟΤχΧε «_________(ΧνΟϊ≥Τ)ΓΘ
Δσ ≤βΕ®Φν ΫΧΦΥαΡχΨßΧεΒΡΉι≥…
ΈΣ≤βΕ®Φν ΫΧΦΥαΡχΨßΧεΘ®xNiCO3yNi(OH)2zH2OΘ©Ήι≥…Θ§Ρ≥–ΓΉι…ηΦΤΝΥ»γœ¬ Β―ιΖΫΑΗΦΑΉΑ÷ΟΘΚ
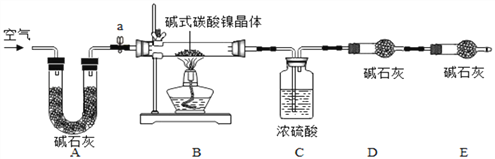
Θ®Ή ΝœΩ®Τ§2Θ©
(1)Φν ΫΧΦΥαΡχΨßΧε ή»»ΜαΆξ»ΪΖ÷Ϋβ…ζ≥…NiOΓΔCO2ΦΑH2O
(2)‘ΎΈ¬Ε»ΩΊ÷Τ≤ΜΒ±ΒΦ÷¬Έ¬Ε»ΙΐΗΏ ±Θ§NiOΜα±Μ―θΜ·≥…Ni2O3
(3)Φν ·Μ“ «NaOHΦΑCaOΒΡΜλΚœΈοΘ§Ω…“‘Έϋ ’CO2ΚΆH2O
Θ® Β―ι≤Ϋ÷ηΘ©
ΔΌΦλ≤ιΉΑ÷ΟΤχΟή–‘ΘΜΔΎΉΦ»Ζ≥Τ»Γ3.77gΦν ΫΧΦΥαΡχΨßΧεΘ®xNiCO3yNi(OH)2zH2OΘ©Ζ≈‘ΎBΉΑ÷Ο÷–Θ§Ν§Ϋ”“«ΤςΘΜΔέ¥ρΩΣΒ·Μ…Φ–aΘ§ΙΡ»κ“ΜΕΈ ±ΦδΩ’ΤχΘ§Ζ÷±π≥ΤΝΩΉΑ÷ΟCΓΔDΓΔEΒΡ÷ ΝΩΘΜΔήΙΊ±’Β·Μ…Φ–aΘ§Φ”»»ΉΑ÷ΟB÷ΝΉΑ÷ΟC÷–ΒΦΙήΡ©ΕΥΈόΤχ≈ίΟΑ≥ωΘΜΔί¥ρΩΣΒ·Μ…Φ–aΘ§ΜΚΜΚΙΡ»κ“ΜΕΈ ±ΦδΩ’ΤχΘΜΔόΖ÷±πΉΦ»Ζ≥ΤΝΩΉΑ÷ΟCΓΔDΓΔEΒΡ÷ ΝΩΘΜΔΏΗυΨί ΐΨίΫχ––ΦΤΥψ(œύΙΊ ΐΨί»γœ¬±μ)ΓΘ
ΉΑ÷ΟC/g | ΉΑ÷ΟD/g | ΉΑ÷ΟE/g | |
Φ”»»«Α | 200.00 | 180.00 | 180.00 |
Φ”»»Κσ | 201.08 | 180.44 | 180.00 |
Θ® Β―ιΖ÷ΈωΦΑ ΐΨί¥ΠάμΘ©
8) Β―ιΙΐ≥Χ÷–≤Ϋ÷ηΔίΙΡ»κΩ’ΤχΒΡΡΩΒΡ «__________________ΓΘ
(9)ΦΤΥψ3.77gΦν ΫΧΦΥαΡχΨßΧεΘ®xNiCO3yNi(OH)2zH2OΘ©÷–Ρχ‘ΣΥΊΒΡ÷ ΝΩ_________(–¥≥ωΦΤΥψΙΐ≥ΧΘ§ΦΤΥψΫαΙϊΉΦ»ΖΒΫ–Γ ΐΒψΚσΝΫΈΜ)ΓΘ
Θ® Β―ιΖ¥ΥΦΘ©
(10)Νμ“Μ–ΓΉιΆ§―ß‘Ύ Β―ι÷–ΖΔœ÷Θ§ Β―ιΫα χΚσΘ§≥ΤΒΟΉΑ÷ΟB÷–≤–ΝτΙΧΧε÷ ΝΩΟςœ‘ΤΪ¥σΘ§άœ Π¥χΝλ»ΪΉιΆ§―ßΨ≠ΙΐΉ–œΗΖ÷ΈωΚσΖΔœ÷Θ§’β «”…”ΎΗΟΉιΆ§―ßΦ”»» ±‘ΎΨΤΨΪΒΤ…œΦ”ΝΥΧζΥΩΆχ’÷Θ§Έ¬Ε»ΙΐΗΏΥυΒΦ÷¬ΓΘ«κΈ ΗΟΉιΆ§―ß≥ΤΒΟΒΡ≤–ΝτΙΧΧε÷–Ρχ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΩ…Ρή «__________ΓΘ
A.70.08% B.75.88% C.78.67% D.79.58%