ƒøƒ⁄»ð
°æƒø°ø–°ª™Õ¨—ߌ™¡À≤‚∂® ت“ Ø—˘∆∑÷–úÀ·∏∆µƒ÷ ¡ø∑÷ ˝£¨»°œýµ»÷ ¡ø(14.0g)µƒŒÂ∑ð—˘∆∑∑÷±∫Õœ°—ŒÀ·∑¥”¶£¨∆‰ µ—È ˝æ𺫬º»Áœ¬±Ì°££®‘”÷ º»≤ª»Ð”⁄ÀÆ“≤≤ª∏˙À·∑¥”¶£ª≤ªøº¬«∂˛—ıªØú‘⁄ÀÆ÷–µƒ»ÐΩ‚£©Õ®π˝∂‘ ˝æðµƒ∑÷Œˆ∫Õ±»Ωœ£¨ªÿ¥œ¬¡–”–πÿŒ £∫
—ŒÀ·µƒ÷ ¡ø(g) | 10 | 20 | 40 | 60 | 80 |
∂˛—ıªØú÷ ¡ø£®g£© | 0.88 | 1.76 | 3.52 | 4.4 | X |
£®1£©Xµƒ ˝÷µŒ™°£
£®2£©7.4g«‚—ıªØ∏∆”ÎgúÀ·∏∆∫¨µƒ∏∆‘™Àÿ÷ ¡øœýµ»£ø
£®3£©«Î‘⁄œ¬Õº÷–ª≠≥ˆ‘⁄14.0g—˘∆∑÷–º”œ°—ŒÀ·µƒ÷ ¡ø”Î≤˙…˙∆¯ÃÂ÷ ¡ø±‰ªØµƒ∫Ø ˝πÿœµ æ“‚Õº°£
£®4£© ت“ Ø—˘∆∑÷–úÀ·∏∆µƒ÷ ¡ø∑÷ ˝ «?£®æ´»∑µΩ0.1%£©
°æ¥∞∏°ø
£®1£©4.4
£®2£©10g
£®3£©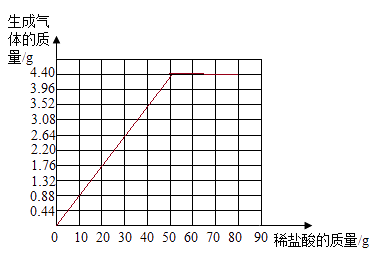
£®4£©71.4%
°æΩ‚Œˆ°ø£®1£©∂‘”⁄ ˝æ𥶿Ì£¨”¶œ»’“≥ˆ ˝æð÷ƺ‰µƒπʬ…£¨»ª∫Û∑¢œ÷πʬ…±ª∆∆ªµ£¨Ω¯∂¯∑÷Œˆ‘≠“Ú£¨∏˘æð±Ì÷– ˝æð£∫º”»Î10g£¨≤˙…˙∂˛—ıªØúµƒ÷ ¡øŒ™0.88g£¨π º”»Î20g£¨≤˙…˙∂˛—ıªØúµƒ÷ ¡øŒ™1.76g £¨µ±º”»Î—ŒÀ·÷ ¡øŒ™60g ±£¨≤˙…˙∂˛—ıªØúµƒ÷ ¡ø”¶Œ™5.28g £¨ø…÷ª…˙≥…4.4g£¨‘ÚÀµ√˜ÃºÀ·∏∆“—æ≠»´≤ø±ªœ˚∫ƒ£¨À˘“‘Xµƒ÷µŒ™4.4£®2£©∏˘æð‘™Àÿµƒ÷ ¡ø=ŒÔ÷ µƒ÷ ¡ø°¡‘™Àÿµƒ÷ ¡ø∑÷ ˝£¨π ø……ËúÀ·∏∆µƒ÷ ¡øŒ™x£¨¡– ΩŒ™£∫7.4g°¡40/74°¡100%=x°¡40/100°¡100%£¨x=10g£®3£©ª≠Õº ±£¨”¶’“◊º2∏ˆµ„£¨“ª «∆ ºµ„£¨∂˛ «◊™’€µ„£¨∆ ºµ„”¶¥”0ø™ º£¨∏˘æð±Ì÷–µƒ ˝æðπʬ…£¨ø…÷™£¨µ±ÃºÀ·∏∆«°∫√±ªÕÍ»´∑¥”¶£¨œ˚∫ƒµƒ—ŒÀ·÷ ¡øŒ™50g£¨π ◊™’€µ„µƒ◊¯±ÍŒ™£∫œ°—ŒÀ·÷ ¡øŒ™50g£¨∂˛—ıªØú÷ ¡øŒ™4.4£¨Õº  ;£®4£©∏˘æðªØ—ß∑¥”¶∑Ω≥Ã Ω£∫CaCO3+2HCl=CaCl2+H2O+CO2°¸÷–CaCO3”ÎCO2µƒ÷ ¡øπÿœµ£¨º¥ø…«Û≥ˆÃºÀ·∏∆µƒ÷ ¡ø£¨Ω¯∂¯º∆À„ ت“ Ø—˘∆∑÷–úÀ·∏∆µƒ÷ ¡ø∑÷ ˝
;£®4£©∏˘æðªØ—ß∑¥”¶∑Ω≥Ã Ω£∫CaCO3+2HCl=CaCl2+H2O+CO2°¸÷–CaCO3”ÎCO2µƒ÷ ¡øπÿœµ£¨º¥ø…«Û≥ˆÃºÀ·∏∆µƒ÷ ¡ø£¨Ω¯∂¯º∆À„ ت“ Ø—˘∆∑÷–úÀ·∏∆µƒ÷ ¡ø∑÷ ˝
Ω‚£∫…ËúÀ·∏∆µƒ÷ ¡øŒ™x
CaCO3+ | 2HCl®T | CaCl2+H2O+ | CO2°¸ |
100 | 44 | ||
x | 4.4g |
100£∫44=x£∫4.4g
X=10g
ت“ Ø—˘∆∑÷–úÀ·∏∆µƒ÷ ¡ø∑÷ ˝=10g/14g°¡100%=71.4%
°æøºµ„æ´Œˆ°ø»œ’Ê…Û£¨ ◊œ»–Ë“™¡ÀΩ‚∏˘æðªØ—ß∑¥”¶∑Ω≥Ã Ωµƒº∆À„(∏˜ŒÔ÷ º‰÷ ¡ø±»=œµ ˝°¡œý∂‘∑÷◊”÷ ¡ø÷Ʊ»)£Æ