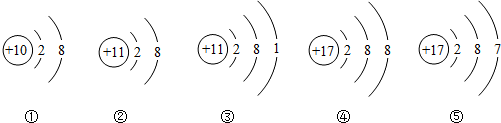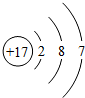��Ŀ����
ij��������Ҫ�ⶨһ����������صķϸֲĵ���ʴ�̶ȣ��ٶ��÷ϸֳɷ�Ϊ���������������ʣ����ʲ�����ˮ�������κη�Ӧ��������������������Ӧ����ȡ����Ʒ289g����һ�ྻ���ձ��У��ٽ�������250��ϡ����ƽ����Ϊ5�μ��뵽�ձ��У����ʵ���������£�
���ձ������ʹ��˵õ���Һ276.8g���Իش��������⣺��ע�������ó���֪ʶ���
��1������������������� ��
��2��������֪�����г����μӷ�Ӧ�ĵ�������������x���ı���ʽ ��
��3��������ϡ�������������������
��4�������Ʒ�б���ʴ����Ԫ��ռԭ�ֲģ�����ʴǰ�ģ�������������ȡ��������
| ϡ������������λ/g�� | 50 | 50 | 50 | 50 | 50 |
| �ձ���ʣ����������������λ/g�� | 78 | 128 | 178 | 227.8 | 277.6 |
��1�������������������
��2��������֪�����г����μӷ�Ӧ�ĵ�������������x���ı���ʽ
��3��������ϡ�������������������
��4�������Ʒ�б���ʴ����Ԫ��ռԭ�ֲģ�����ʴǰ�ģ�������������ȡ��������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����������غ㶨�ɣ���Ӧǰ�����ʵ�������֮��Ϊ���ɵ������������
��2�������������ᷴӦ�Ļ�ѧ����ʽ�������������б���ʽ��
��3�������������ᷴӦ�Ļ�ѧ����ʽ�����������������������Ӧ���������������������������
��4�������������е���Ԫ��Ϊ����ʴ����Ԫ�ؽ��м��㣮
��2�������������ᷴӦ�Ļ�ѧ����ʽ�������������б���ʽ��
��3�������������ᷴӦ�Ļ�ѧ����ʽ�����������������������Ӧ���������������������������
��4�������������е���Ԫ��Ϊ����ʴ����Ԫ�ؽ��м��㣮
����⣺
��1�������������������250g+28g-277.6g=0.4g��
��2����μӷ�Ӧ�ĵ�����������������Ϊx
Fe+H2SO4=H2��+FeSO4
56 2
x 0.4g
=
x=11.2g��
��ʵ�����ݿ�֪��������Ӧ��ϡ���������Ϊ100g
��������Ӧ��ϡ���������ʵ�����Ϊy
Fe+H2SO4=H2��+FeSO4
98 2
y 0.4g
=
y=19.6g
����ϡ������������������ǣ�
��100%=19.6%��
��4��ԭ�ֲ�������������ɣ�
���ʵ�����=277.6g-276.8g=0.8g��
�ϸֲ�����������Ϊ11.2g��
��ϸֲ���������������=28g-11.2g-0.8g=16g��
����ʴ����Ԫ�ص�����Ϊ��16g��
��100%=11.2g��
��Ʒ�б���ʴ����Ԫ��ռԭ�ֲģ�����ʴǰ�ģ�����������Ϊ��
��100%��48%��
�𰸣�
��1��0.4g
��2��
=
��3������ϡ�����������������19.6%
��4������Ʒ�б���ʴ����Ԫ��ռԭ�ֲģ�����ʴǰ�ģ�����������48%
��1�������������������250g+28g-277.6g=0.4g��
��2����μӷ�Ӧ�ĵ�����������������Ϊx
Fe+H2SO4=H2��+FeSO4
56 2
x 0.4g
| 56 |
| 2 |
| x |
| 0.4g |
x=11.2g��
��ʵ�����ݿ�֪��������Ӧ��ϡ���������Ϊ100g
��������Ӧ��ϡ���������ʵ�����Ϊy
Fe+H2SO4=H2��+FeSO4
98 2
y 0.4g
| 98 |
| 2 |
| y |
| 0.4g |
y=19.6g
����ϡ������������������ǣ�
| 19.6g |
| 100g |
��4��ԭ�ֲ�������������ɣ�
���ʵ�����=277.6g-276.8g=0.8g��
�ϸֲ�����������Ϊ11.2g��
��ϸֲ���������������=28g-11.2g-0.8g=16g��
����ʴ����Ԫ�ص�����Ϊ��16g��
| 56��2 |
| 56��2+16��3 |
��Ʒ�б���ʴ����Ԫ��ռԭ�ֲģ�����ʴǰ�ģ�����������Ϊ��
| 11.2g |
| 11.2g+11.2g+0.8g |
�𰸣�
��1��0.4g
��2��
| 56 |
| 2 |
| x |
| 0.4g |
��3������ϡ�����������������19.6%
��4������Ʒ�б���ʴ����Ԫ��ռԭ�ֲģ�����ʴǰ�ģ�����������48%
�������������Ĺؼ���Ҫ֪���ϸֲ��б���ʴ����Ԫ�ء�δ����ʴ����Ԫ���Լ����ʵ�������

��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
�����Ŀ
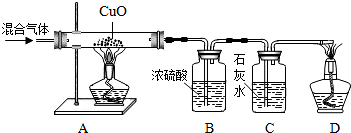
 A��B��C��D�ֱ���������þ��ϡ���ᡢп�ۡ��Ȼ�ͭ��Һ�е�һ�����ʣ�ijͬѧ���ڹ��ɼ������ʵĻ�ѧ���ʣ�������������������
A��B��C��D�ֱ���������þ��ϡ���ᡢп�ۡ��Ȼ�ͭ��Һ�е�һ�����ʣ�ijͬѧ���ڹ��ɼ������ʵĻ�ѧ���ʣ�������������������